
HL Paper 3
Heavy metal ions are an important environmental concern.
State the name of one method, other than precipitation, of removing heavy metal ions from solution in water.
The solubility product, Ksp , of cadmium sulfide, CdS, is 8.0 × 10–27. Determine the concentration of cadmium ions in 1.0 dm3 of a saturated solution of cadmium sulfide to which 0.10 mol of solid sodium sulfide has been added, stating any assumption you make.
Metal ions may cause unwanted environmental effects.
The presence of iron(III) ions can catalyse the formation of hydroxyl radicals from O2− and H2O2 in the Haber–Weiss reaction. State the equations for this process.
Zinc ions, toxic to aquatic life, may be removed by adding a solution containing hydroxide ions. Determine the concentration of zinc ions in a saturated solution of zinc hydroxide at 298K using information from section 32 of the data booklet.
Aluminium is produced by the electrolysis of a molten electrolyte containing bauxite.
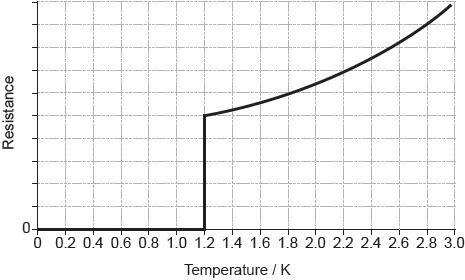
The graph of the resistance of aluminium with temperature is shown below.
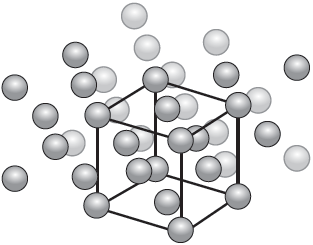
The diagram illustrates the crystal structure of aluminium metal with the unit cell indicated. Outline the significance of the unit cell.
When X-rays of wavelength 0.154 nm are directed at a crystal of aluminium, the first order diffraction pattern is observed at 18°. Determine the separation of layers of aluminium atoms in the crystal, in m, using section 1 of the data booklet.
Deduce what the shape of the graph indicates about aluminium.
Outline why the resistance of aluminium increases above 1.2 K.
The concentration of aluminium in drinking water can be reduced by precipitating aluminium hydroxide. Calculate the maximum concentration of aluminium ions in water of pH 7 at 298 K. Solubility product of aluminium hydroxide = 3.3 × 10−34 at 298 K.
Rhodium and palladium are often used together in catalytic converters. Rhodium is a good reduction catalyst whereas palladium is a good oxidation catalyst.
Nickel(II) ions are least soluble at pH 10.5. Calculate the molar solubility of nickel(II) hydroxide at this pH. KspNi(OH)2 = 5.48 × 10–16.
Rhodium is paramagnetic with an electron configuration of [Kr] 5s14d8.
Explain, in terms of electron spin pairing, why paramagnetic substances are attracted to a magnetic field and diamagnetic substances are not.
Rhodium is a type 1 superconductor.
Sketch graphs of resistance against temperature for a conductor and superconductor.
Contrast type 1 and type 2 superconductors by referring to three differences between them.
Both HDPE (high density polyethene) and LDPE (low density polyethene) are produced by the polymerization of ethene.
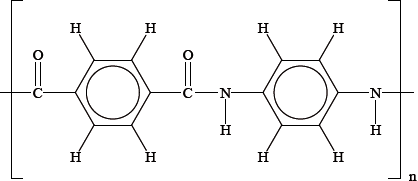
An alternative method of polymerizing molecules is condensation polymerization. One of the earliest condensation polymers was nylon-6. A short section of the polymer chain of nylon-6 is shown below.
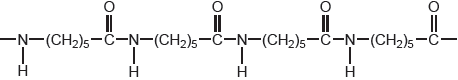
Draw the structure of the monomer from which nylon-6 is produced by a condensation reaction.
Deduce, giving a reason, whether the atom economy of a condensation polymerization, such as this, would be greater or less than an addition polymerization, such as the formation of HDPE.
Vanadium forms a body centred cubic (BCC) crystal structure with an edge length of 303 pm, (303 × 10−12 m).
Deduce the number of atoms per unit cell in vanadium.
Calculate the expected first order diffraction pattern angle, in degrees, if x-rays of wavelength 150 pm are directed at a crystal of vanadium. Assume the edge length of the crystal to be the same as separation of layers of vanadium atoms found by x-ray diffraction. Use section 1 of the data booklet.
Calculate the average mass, in g, of a vanadium atom by using sections 2 and 6 of the data booklet.
Determine the volume, in cm3, of a vanadium unit cell.
Determine the density, in g cm−3, of vanadium by using your answers to (a)(i), (a)(iii) and (a)(iv).
Vanadium and other transition metals can interfere with cell metabolism.
State and explain one process, other than by creating free radicals, by which transition metals interfere with cell metabolism.
Vanadium(IV) ions can create free radicals by a Fenton reaction.
Deduce the equation for the reaction of V4+ with hydrogen peroxide.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Describe how the monomers of addition polymers and of condensation polymers differ.
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
Lanthanum has a hexagonal close packed (hcp) crystal structure. State the coordination number of each lanthanum atom.
Lanthanum becomes superconducting below 5 K. Explain, in terms of Bardeen–Cooper–Schrieffer (BCS) theory, how superconductivity occurs.
Outline why superconductivity only occurs at low temperatures.
The development of materials with unique properties is critical to advances in industry.
Explain why Type 2 superconductors are generally more useful than Type 1.
Kevlar behaves as a lyotropic liquid crystal when dissolved in suitable solvents. Its structure is shown below.
State the properties that a molecule, such as Kevlar, must have in order to enable it to behave as a liquid crystal.
Discuss the additional properties that a substance must have to make it suitable for commercial liquid-crystal displays.
Explain what is meant by the term lyotropic.
Waste water can contain metal ions such as chromium. Chromium ions can cause damage to the liver and kidneys. Chromium ions can be removed from water by chemical precipitation using hydroxide ions.
Assuming chromium is present as \({\text{C}}{{\text{r}}^{3 + }}\), state an equation for its reaction with hydroxide ions, include state symbols.
State an expression for the solubility product constant, \({K_{{\text{sp}}}}\), for chromium(III) hydroxide.
The solubility product of chromium(III) hydroxide is \(1.00 \times {10^{ - 33}}{\text{ mo}}{{\text{l}}^{\text{4}}}{\text{d}}{{\text{m}}^{ - 12}}\) at 298 K. Calculate the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of \({\text{C}}{{\text{r}}^{3 + }}\) in the solution, when chromium(III) hydroxide is precipitated.
One method of removing heavy metal ions from a solution is by precipitation.
State an ionic equation, including state symbols, for the reaction taking place when an aqueous solution containing chloride ions is added to an aqueous solution containing lead(II) ions.
The solubility product, \({K_{{\text{sp}}}}\), of lead(II) chloride is \(1.7 \times {10^{ - 5}}\) at 298 K. Determine the concentration of lead(II) ions, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), when equal volumes of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous potassium chloride and a solution of \({\text{0.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) lead(II) ions are mixed.
State any assumption used.
Heavy-metal ions such as \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) are often present in waste water sewage. The \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) ions can be removed from the sewage by means of chemical precipitation.
State an expression for the solubility product constant, \({K_{{\text{sp}}}}\), for copper(II) hydroxide.
The solubility product of copper(II) hydroxide is \(4.8 \times {10^{ - 20}}\) at a given temperature.
Determine the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) in the solution when copper(II) hydroxide is precipitated.
Iron ore can be reduced in a blast furnace.
The properties of a metal can be altered by alloying or heat treatment. Explain why alloying can modify the structure and properties of a metal.
Chemical vapour deposition (CVD) produces multi-walled carbon nanotubes (MWCNT) of a more appropriate size for use in liquid crystals than production by arc discharge.
MWCNT are very small in size and can greatly increase switching speeds in a liquid crystal allowing the liquid crystal to change orientation quickly.
Discuss two other properties a substance should have to be suitable for use in liquid crystal displays.
Distinguish between thermotropic liquid crystals and lyotropic liquid crystals.
Thermotropic:
Lyotropic:
Two substances that can be used in liquid crystals are commonly called PAA (4-azoxydianisole) and 5CB (4-pentyl-\({\text{4'}}\)-cyanobiphenyl).
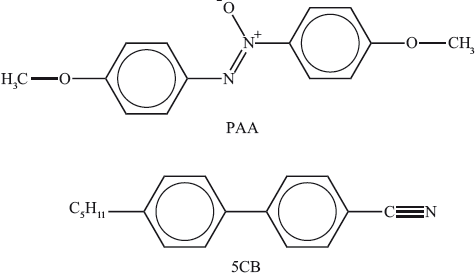
Discuss on the molecular level three different factors that explain their liquid-crystal properties.
Explain the workings of liquid crystals made up of compounds such as 5CB in liquid-crystal displays.
Antimony and its compounds are toxic, so it is important to check that the catalyst is removed from the final product. One technique to detect antimony is Inductively Coupled Plasma Mass Spectroscopy (ICP-MS).
Outline the nature of the plasma state and how it is produced in ICP-MS.
Hydrogen sulfide could be used to remove antimony(III) ions from a solution.
Determine the concentration of antimony(III) ions that would be required to precipitate antimony(III) sulfide in a solution saturated with hydrogen sulfide.
[S2−] in water saturated with hydrogen sulfide = 1.0 × 10−14 mol dm−3
Ksp (Sb2S3) = 1.6 × 10−93
Identify a ligand that could be used to chelate antimony(III) ions in solution.
Polymers can be classified as addition polymers or condensation polymers.
Outline the difference in the way in which polymerization occurs, stating a specific example of a polymer produced by each process.
Polymers can either soften when heated or remain rigid until they decompose or combust. Other than Kevlar, state the names of one polymer that softens and one that does not. Explain this difference on a molecular level.
Softening polymer:
Non-softening polymer:
Explanation:
Raw sewage is the water-carried waste that flows away from a community. If it is discharged untreated into rivers and the sea it causes pollution. Therefore, waste water should be treated before it is discharged.
Phosphate ions are one of the pollutants removed from sewage water by chemical precipitation using calcium ions.
The solubility product, \({K_{{\text{sp}}}}\), of calcium phosphate, \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}\), is \(1.20 \times {10^{ - 26}}\) at 298 K.
Determine the concentration of phosphate ions, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), in a saturated solution of calcium phosphate.
Kevlar® is a lyotropic liquid crystal. Explain the strength of Kevlar® and its solubility in concentrated sulfuric acid.
Describe the use of silicon in photovoltaic cells. Include the following in your description:
• why pure silicon is a better conductor than non-metals such as sulfur and phosphorus
• how a p-type semiconductor made from silicon is different from pure silicon
• how sunlight interacts with semiconductors.
Propene can polymerize to form polypropene.
Propene monomer: 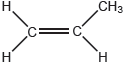
Distinguish between the manufacture of polyester and polyethene.
Antioxidants can be used to prolong the shelf life of food.
\({{\text{(EDTA)}}^{4 - }}\), the ethylenediaminetetraacetate anion, is a chelate ligand with the following structure.
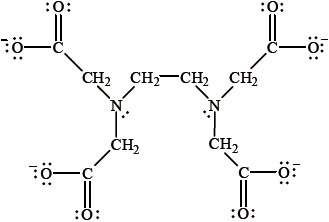
It has been found to inhibit the \({\text{F}}{{\text{e}}^{2 + }}\) catalysed oxidation of raw beef. Explain why \({{\text{(EDTA)}}^{4 - }}\) can be described as a chelate ligand.
Chlorofluorocarbons, CFCs, deplete the ozone layer.
Chlorine atoms and nitrogen oxides react at the surface of ice particles in the arctic winter.
State the equations that represent the depletion of ozone in the stratosphere which is catalysed by chlorine free radicals.
(i) Deduce the type of catalysis that occurs.
(ii) Outline why the depletion of ozone is greatest during the arctic spring.
In order to make waste water acceptable for drinking, it is treated in a series of steps to remove hazardous substances.
Tertiary treatment removes phosphates, nitrates and heavy metal ions from water.
The solubility product constant, \({K_{{\text{sp}}}}\), of cadmium(II) sulfide, CdS, is \({\text{8.00}} \times {\text{1}}{{\text{0}}^{ - 28}}\) at 298 K. Determine the concentration of cadmium(II) ions, \({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}}\), in a saturated solution of cadmium(II) sulfide.
Explain how the addition of hydrogen sulfide gas can decrease the concentration of cadmium(II) ions in a saturated solution.
Compounds of heavy metals are one type of toxic substance found in water. Lead(II) ions, \({\text{P}}{{\text{b}}^{2 + }}\), can be removed by bubbling hydrogen sulfide, \({{\text{H}}_{\text{2}}}{\text{S}}\), through polluted water. The solubility product of lead sulfide is \({\text{1.25}} \times {\text{1}}{{\text{0}}^{ - 28}}{\text{ mo}}{{\text{l}}^{\text{2}}}{\text{d}}{{\text{m}}^{ - 6}}\) at 25 °C.
Calculate the concentration of \({\text{P}}{{\text{b}}^{2 + }}\) ions in a saturated solution of lead sulfide.
Explain how the addition of hydrogen sulfide decreases the concentration of \({\text{P}}{{\text{b}}^{2 + }}\) ions in a saturated solution.
Polymers, used extensively worldwide, are large molecular mass substances consisting of repeating monomer units.
State the type of mechanism occurring in the manufacture of low-density poly(ethene).
Distinguish between addition and condensation polymers in terms of how the monomers react together.
Describe and explain how the properties of condensation polymers depend on three structural features.
Liquid-crystal displays are used in digital watches, calculators and laptops.
Kevlar is a condensation polymer that is often used in liquid-crystal displays. A section of the polymer is shown below.
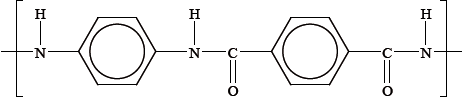
Explain the strength of Kevlar in terms of its structure and bonding.
Explain why a bullet-proof vest made of Kevlar should be stored away from acids.
Since the accidental discovery of polyethene in the 1930s, polymers have played an essential role in daily life because of their wide range of properties and uses.
Polyurethanes are made from dialcohol (diol) and diisocyanate monomers. By considering the structures of the two monomers and the repeating unit of the polymer given below, suggest why it could be argued that this reaction is not an example of a condensation polymer.
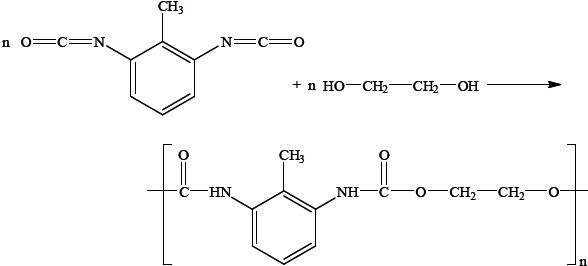
Kevlar is another example of a condensation polymer. Explain how the great strength of Kevlar depends on its structure.
Heavy metal ions are pollutants that can be removed in the tertiary stage of waste water treatment.
A water sample at 25 °C contains lead and sulfate ions in the following concentrations:
\[\begin{array}{*{20}{l}} {[{\text{P}}{{\text{b}}^{2 + }}] = 2.32 \times {{10}^{ - 6}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}} \\ {[{\text{SO}}_4^{2 - }] = 4.15 \times {{10}^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}} \end{array}\]
The solubility product constant, \({K_{{\text{sp}}}}\), of lead sulfate is \(1.80 \times {10^{ - 8}}\) at 25 °C.
State the expression for the solubility product constant, \({K_{{\text{sp}}}}\), of lead sulfate.
Deduce why lead sulfate will not precipitate out of the water sample at these concentrations.
Some magnesium sulfate is added to the water sample. Determine the increase in sulfate ion concentration needed for lead sulfate to precipitate.
Ethene is one of the major products of this process and much of it is converted to polyethene using the Ziegler-Natta process. State the catalysts used and the ways in which the conditions of this process differ from the free-radical polymerization process.
Chemistry has made a significant contribution to the development of liquid-crystal displays (LCDs).
The diagram below is a representation of an LCD. The planes of polarization of the analyser and the polarizer are at right angles to each other.
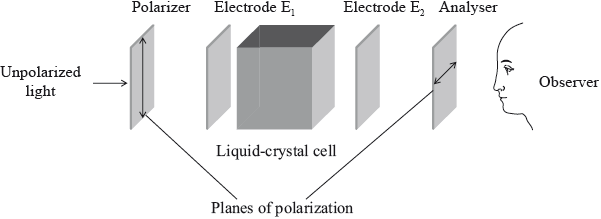
State what the observer would see if the liquid crystal was not present and there was no voltage between the electrodes \({{\text{E}}_{\text{1}}}\) and \({{\text{E}}_{\text{2}}}\).
Explain how the addition of a liquid crystal to the cell changes what the observer sees.
Explain how the application of an electric field between the electrodes, \({{\text{E}}_{\text{1}}}\) and \({{\text{E}}_{\text{2}}}\), changes what the observer sees in b (i).
The molecule below has liquid-crystal display properties.
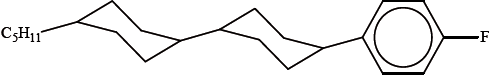
Suggest two reasons why the molecule is suitable for use in liquid-crystal display devices.
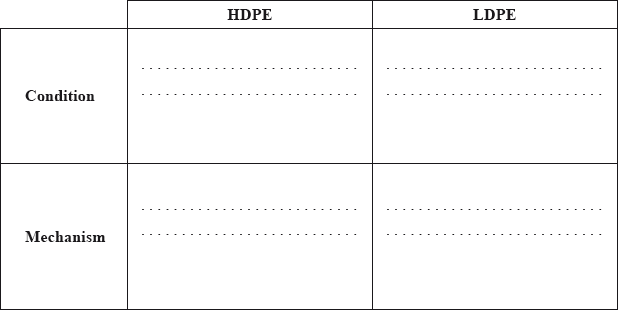
Ethene can be polymerized to form poly(ethene) and, depending on the conditions used, either high-density poly(ethene) (HDPE) or low-density poly(ethene) (LDPE) is formed.
(i) Other than density, state two differences in the physical properties of HDPE and LDPE.
(ii) Outline how the differences in (a)(i) relate to differences in their chemical structure.
State the conditions required to produce HDPE and LDPE and the name of each type of mechanism involved.
Kevlar can be made by reacting 1,4-diaminobenzene, \({{\text{H}}_{\text{2}}}{\text{N}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{N}}{{\text{H}}_{\text{2}}}\), with 1,4-benzenedicarbonyl chloride, \({\text{ClOC}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{COCl}}\). Write the equation for the reaction of n molecules of 1,4-diaminobenzene reacting with n molecules of 1,4-benzenedicarbonyl chloride.
Kevlar is an example of a lyotropic liquid crystal. Outline what is meant by lyotropic liquid crystal.
\[{\text{n }}{{\text{H}}_2}{\text{N}}{{\text{C}}_6}{{\text{H}}_4}{\text{N}}{{\text{H}}_2} + {\text{n ClOC}}{{\text{C}}_6}{{\text{H}}_4}{\text{COCl}} \to {\rm{H\rlap{-} [HN}}{{\text{C}}_6}{{\text{H}}_4}{\text{NHOC}}{{\text{C}}_6}{{\text{H}}_4}{\text{CO}}{{\rm{\rlap{-} ]}}_{\text{n}}}{\text{Cl}} + {\text{2(n}} - {\text{1)HCl}}\]
The following diagram shows two adjacent molecules in a sample of solid Kevlar.
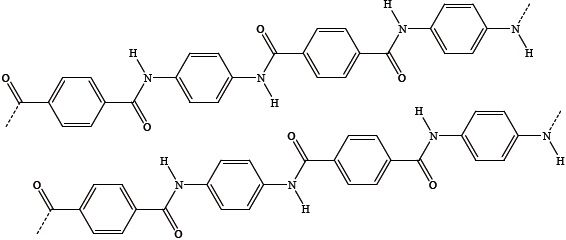
Kevlar is very unreactive but dissolves in concentrated sulfuric acid.
State the type of polymerization involved.
(i) Identify the strongest type of intermolecular force between the two molecules.
(ii) Annotate the diagram (above) by adding dotted lines to show the strongest intermolecular forces.
Kevlar is five times as strong as steel, partly due to its strong intermolecular forces. State another feature of the molecules which gives Kevlar such great strength.
(i) Suggest how the sulfuric acid is able to separate the Kevlar chains.
(ii) Evaluate the long-term environmental impact of Kevlar.
Another polymer that has cross-linking is Kevlar. Kevlar can be made by reacting 1,4-diaminobenzene with benzene-1,4-dicarboxylic acid.
Draw the structural formula of the repeating unit in Kevlar.
Explain how the long rigid chains in Kevlar are able to form cross-links to build up a three-dimensional structure.
Industrial effluent is found to be highly contaminated with silver and lead ions. A sample of water contains \(8.0 \times {10^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ A}}{{\text{g}}^ + }\) and \(1.9 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ P}}{{\text{b}}^{2 + }}\). On the addition of chloride ions both \({\text{AgCl }}({K_{sp}} = 1.8 \times {10^{ - 10}})\) and \({\text{PbC}}{{\text{l}}_{\text{2}}}{\text{ }}({K_{sp}} = 1.7 \times {10^{ - 5}})\) precipitate from the solution. Determine the concentration of \({\text{C}}{{\text{l}}^ - }\) needed to initiate the precipitation of each salt and deduce which salt precipitates first.
Polymers are made up of repeating monomer units which can be manipulated in various ways to give structures with desired properties.
Fermentation of sugars from corn starch produces propane-1,3-diol, which can be polymerized with benzene-1,4-dicarboxylic acid to produce the PTT polymer (polytrimethylene terephthalate).
(i) Draw the molecular structure of each monomer.
(ii) Deduce the name of the linkage formed on polymerization between the two monomers and the name of the inorganic product.
The structure of 4-pentyl-4-cyanobiphenyl, a commercially available nematic crystalline material used in electrical display devices, is shown below.
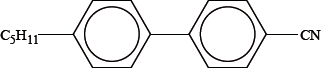
Explain how the three different parts of the molecule contribute to the properties of the compound used in electrical display devices.
CN:
\({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}\):
 :
:
Describe and explain in molecular terms the workings of a twisted nematic liquid crystal.
Liquid crystals are widely used in devices such as calculators, laptop computers and advanced optical materials.
Kevlar® is a material used in bullet-proof vests.
(i) Deduce the products formed by a condensation polymerization reaction of the monomers benzene-1,4-diamine and benzene-1,4-dicarbonyl chloride to form Kevlar®.
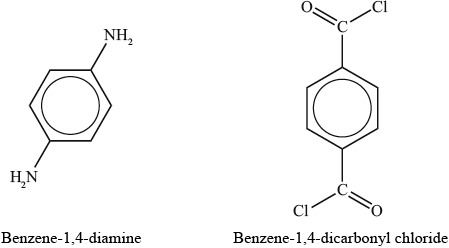
(ii) Describe the factors which account for the inherent strength of Kevlar®.
(iii) Outline why Kevlar® can dissolve in concentrated sulfuric acid.
(a) State one other example of a lyotropic liquid crystal and describe the difference between lyotropic and thermotropic liquid crystals.
(b) Name a thermotropic liquid crystal.
(c) Explain the liquid-crystal behaviour of the thermotropic liquid crystal named in part (b), on the molecular level.
Heavy metal ions can be removed by adding hydroxide ions. When hydroxide ions are added to a solution containing nickel ions, a precipitate of nickel(II) hydroxide, \({\text{Ni(OH}}{{\text{)}}_{\text{2}}}\), is formed. The solubility product of nickel(II) hydroxide is \(6.50 \times {10^{ - 18}}\) at 298 K. Determine the mass of nickel ions that remains in one litre (\({\text{1.00 d}}{{\text{m}}^{\text{3}}}\)) of water at 298 K with a pH of 7 after the precipitation reaction has occurred.
Aluminium is chemically reactive so it has to be extracted by the electrolysis of aluminium oxide dissolved in molten cryolite.
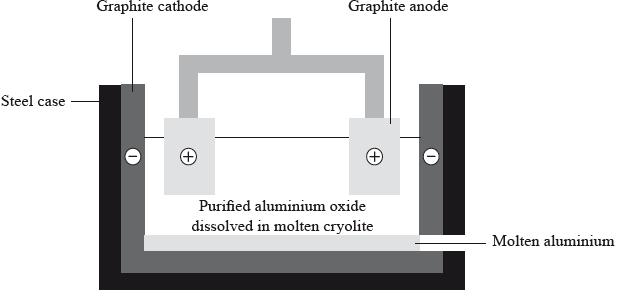
(i) Suggest why the aluminium oxide is dissolved in molten cryolite.
(ii) Deduce an equation for the discharge of the ions at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(iii) Suggest why the anodes have to be replaced at regular intervals.
(i) Outline why aluminium is alloyed with copper and magnesium when used to construct aircraft bodies.
(ii) State two properties of aluminium that make it suitable for use in overhead power cables.
The oxygen levels in water can change for a number of reasons.
The use of phosphate fertilizers can also produce changes in the oxygen concentrations in a river.
Phosphate ions can be removed from a solution by adding calcium ions. State the ionic equation for the reaction of calcium ions with phosphate ions.
Deduce the expression for the solubility product constant, \({K_{{\text{sp}}}}\), of calcium phosphate.
The solubility product of calcium phosphate is \({\text{2.07}} \times {\text{1}}{{\text{0}}^{ - 33}}\) at 298 K. Determine the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of calcium ions, \({\text{C}}{{\text{a}}^{2 + }}\), in a saturated aqueous solution of calcium phosphate.
Poly(propene) has different forms. Isotactic poly(propene) is tough, while atactic poly(propene) is flexible.
Polyethylene terephthalate (PET), represented below, is an example of a condensation polymer.
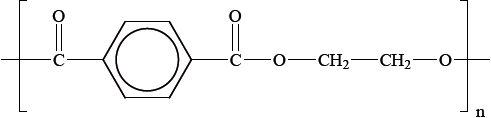
Draw the structures of the monomers that form polyethylene terephthalate.
Predict whether polyethylene terephthalate or isotactic poly(propene) has a higher melting point. Explain your answer in terms of intermolecular forces.
Modern liquid crystals have a structure similar to this biphenyl nitrile.
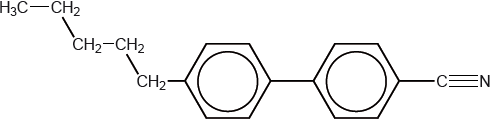
Explain how the structure of biphenyl nitriles makes them suitable for use in liquid-crystal devices.
Liquid crystals are widely used in displays.
Describe the meaning of the term liquid crystals.
When a liquid-crystal display is warmed with a hairdryer, the display loses its clarity and may no longer be visible. Explain why this happens on a molecular level.
Polyethene is the world’s most widely used polymer. It can exist in two forms with distinctive physical properties.
The manufacture of low-density polyethene (LDPE) is initiated by the introduction into ethene of an organic peroxide, ROOR, which, at high temperature and pressure, forms free radicals.
\(ROOR \to 2RO \bullet \)
Polyacrylonitrile is an important polymer used in the manufacture of carbon fibres. The monomer has the structure below.
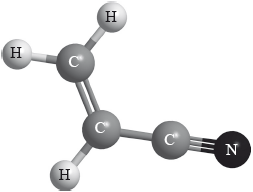
Polyacrylonitrile is similar to polypropene and can exist in two forms.
Draw the structure of the isotactic form of polyacrylonitrile showing three repeating units.
Explain why the isotactic form is more suitable for the manufacture of strong fibres.
Thermotropic liquid crystals are widely used in display devices and sensors.
Describe and explain, in molecular terms, the workings of a twisted nematic liquid crystal.
Aluminium is produced by the electrolysis of aluminium oxide.
State how a low operating temperature is achieved when aluminium oxide is electrolysed.
Liquid crystals are an important component in many devices considered essential in modern life, such as smartphones.
Describe the meaning of the term liquid crystal.
List two properties needed for a substance to be used in a liquid-crystal display.
Soil degradation is a global problem that can lead to a reduction in food production.
Aluminium and magnesium ions are commonly found in different forms in soil. Magnesium ions are important for plant growth, but aluminium ions may be toxic if absorbed by plants. Both these ions can be precipitated in the soil by the formation of their hydroxides. The \({K_{{\text{sp}}}}\) values for magnesium hydroxide and aluminium hydroxide at 298 K are \(1.80 \times {10^{ - 11}}\) and \(3.00 \times {10^{ - 34}}\), respectively.
Determine the concentration of the magnesium and hydroxide ions in a saturated solution of magnesium hydroxide at 298 K, and calculate its pH. Assume there are no other ions present.
Deduce, with a reason, whether the pH of a saturated solution of aluminium hydroxide, at the same temperature, would be greater or less than your answer to (i).
EDTA is produced by reacting ethane-1,2-diamine with chloroethanoic acid, ClCH2COOH.
Identify the other product formed.
Explain why EDTA, a chelating agent, is more effective in removing heavy metal ions from solution than monodentate ligands.
Superconductors are materials that conduct electric current with practically zero resistance.
Describe the Meissner effect.
Outline one difference between type 1 and type 2 superconductors.
Chromium forms coloured compounds and is used to make stainless and hard steel. The distance between layers of chromium atoms in the metal can be obtained using X-ray crystallography.
(i) The diagram below shows the diffraction of two X-ray beams, y and z of wavelength λ, shining on a chromium crystal whose planes are a distance d nm apart.
Deduce the extra distance travelled by the second beam, z, compared to the first one, y.
(ii) State the Bragg’s condition for the observed diffraction to be at its strongest (constructive interference).
(i) The mass of one unit cell of chromium metal is 17.28 × 10−23 g. Calculate the number of unit cells in one mole of chromium. Ar(Cr) = 52.00.
(ii) Deduce the number of atoms of chromium per unit cell.
Antimony oxide is widely used as a homogeneous catalyst for the reaction of benzene-1,4-dicarboxylic acid with ethane-1,2-diol in the production of polyethylene terephthalate (PETE).
Deduce the repeating unit of the polymer and the other product of the reaction.
State the class of polymer to which PETE belongs.
Iron may be extracted from an ore containing Fe2O3 in a blast furnace by reaction with coke, limestone and air. Aluminium is obtained by electrolysis of an ore containing Al2O3.
(i) Outline the cause of electrical resistance in metallic conductors.
(ii) The resistance of two metals was measured as a function of temperature. The following graph was obtained.
Explain the behaviour of metal II below temperature X in terms of the Bardeen–Cooper–Schrieffer (BCS) theory.
(i) Polonium metal has a simple cubic structure. Construct a unit cell diagram and state the coordination number of each atom.
(ii) X-ray diffraction was carried out on polonium using radiation with a wavelength of 8.80×10−11 m. The first order maximum in the diffraction pattern was observed at an angle of 13.0°. Determine the distance, in m, between layers of polonium atoms using section 1 of the data booklet.
Metals have various crystal structures. Cobalt forms a face-centred cubic (FCC) lattice. Two representations of FCC are shown.
Calculate the total number of cobalt atoms within its unit cell.
The atomic radius, r, of cobalt is 1.18 × 10–8 cm. Determine the edge length, in cm, of the unit cell, a, using the second diagram.
Determine a value for the density of cobalt, in g cm–3, using data from sections 2 and 6 of the data booklet and your answers from (a) and (b) (i).
If you did not obtain an answer to (b) (i), use 3.00 × 10–8 cm but this is not the correct answer.
Biphenyl nitriles, such as the molecule shown below, were the first thermotropic liquid crystal molecules to be synthesized.
(i) The monomers from which Kevlar® is produced are given below.
Deduce the formula of the repeating unit of Kevlar®.
(ii) State the structural feature of Kevlar® that is primarily responsible for its strength.
Polymer nanocomposites often have better structural performance than conventional materials. Lithographic etching and metal coordination are two methods of assembling these nanocomposites.
Dendrimers are highly branched nanoparticles with a wide range of usage. One such dendrimer is PAMAM, or polyamidoamine.
The first step in the synthesis is to make the core by reacting ethane-1,2-diamine with methylpropenoate.
Estimate the atom economy of this first step.
Suggest, giving one reason, whether this is an addition or condensation reaction.
Subsequent steps proceed under differing conditions, forming the dendrimer polymer with the following repeating unit.
State the name of one functional group in this repeating unit.
The Fenton and Haber–Weiss reactions convert organic matter in waste water to carbon dioxide and water.
Compare and contrast the Fenton and Haber–Weiss reaction mechanisms.
Adsorption and chelation are two methods of removing heavy metal ion pollution from the environment.
(i) Describe the process of adsorption.
(ii) Deduce the structure of the complex ion formed by the reaction of three H2N−CH2−CH2−NH2 chelating molecules with a mercury(II) ion.