
SL Paper 1
Which sample has the greatest mass?
A. 1 mol of \({\text{S}}{{\text{O}}_{\text{2}}}\)
B. 2 mol of \({{\text{N}}_{\text{2}}}{\text{O}}\)
C. 2 mol of Ar
D. 4 mol of \({\text{N}}{{\text{H}}_{\text{3}}}\)
What is the total number of atoms in 0.100 mol of \({\text{[Pt(N}}{{\text{H}}_3}{{\text{)}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\)?
A. 11
B. \(6.02 \times {10^{22}}\)
C. \(3.01 \times {10^{23}}\)
D. \(6.62 \times {10^{23}}\)
Nitroglycerine, \({{\text{C}}_3}{{\text{H}}_5}{{\text{N}}_3}{{\text{O}}_{\text{9}}}\), can be used in the manufacture of explosives. What is the coefficient of \({{\text{C}}_3}{{\text{H}}_5}{{\text{N}}_3}{{\text{O}}_9}({\text{l)}}\) when the equation for its decomposition reaction is balanced using the lowest whole numbers?
___ \({{\text{C}}_3}{{\text{H}}_5}{{\text{N}}_3}{{\text{O}}_9}{\text{(l)}} \to \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}} + \) ___ \({{\text{H}}_2}{\text{O(l)}} + \) ___ \({{\text{N}}_2}{\text{(g)}} + \) ___ \({{\text{O}}_2}{\text{(g)}}\)
A. 2
B. 4
C. 20
D. 33
4.00 mol of a hydrocarbon with an empirical formula of \({\text{C}}{{\text{H}}_{\text{2}}}\) has a mass of 280 g. What is the molecular formula of this compound?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\)
D. \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}\)
The volume occupied by one mole of an ideal gas at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\) is \({\text{22.4 d}}{{\text{m}}^{\text{3}}}\). What volume, in \({\text{d}}{{\text{m}}^{\text{3}}}\), is occupied by 3.20 g \({{\text{O}}_{\text{2}}}{\text{(g)}}\) at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\)?
A. 2.24
B. 4.48
C. 22.4
D. 71.7
\({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) of an ideal gas at 100 kPa and 25 °C is heated to 50 °C at constant pressure. What is the new volume in \({\text{d}}{{\text{m}}^{\text{3}}}\)?
A. 0.50
B. 0.90
C. 1.1
D. 2.0
In which mixture is NaOH the limiting reagent?
A. 0.20mol NaOH + 0.10mol H2SO4
B. 0.10mol NaOH + 0.10mol H2SO4
C. 0.20mol NaOH + 0.10mol HNO3
D. 0.10mol NaOH + 0.10mol HNO3
What is the percentage yield when 2.0 g of ethene, C2H4, is formed from 5.0 g of ethanol, C2H5OH?
Mr(ethene) = 28; Mr(ethanol) = 46
A. \(\frac{{2.0}}{{28}} \times \frac{{5.0}}{{46}} \times 100\)
B. \(\frac{{\frac{{2.0}}{{28}}}}{{\frac{{5.0}}{{46}}}} \times 100\)
C. \(\frac{{28}}{{2.0}} \times \frac{{5.0}}{{46}} \times 100\)
D. \(\frac{{\frac{{28}}{{2.0}}}}{{\frac{{5.0}}{{46}}}} \times 100\)
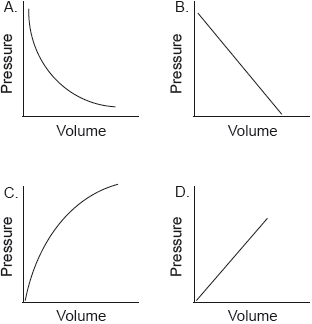
Which graph shows the relationship between the volume and pressure of a fixed mass of an ideal gas?
How many molecules are present in a drop of ethanol, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\), of mass \(2.3 \times {10^{ - 3}}{\text{ g}}\)? \(({{L}} = 6.0 \times {10^{23}}{\text{ mo}}{{\text{l}}^{ - 1}})\)
A. \(3.0 \times {10^{19}}\)
B. \(3.0 \times {10^{20}}\)
C. \(6.0 \times {10^{20}}\)
D. \(6.0 \times {10^{26}}\)
What is the total number of nitrogen atoms in two mol of \({\text{N}}{{\text{H}}_{\text{4}}}{\text{N}}{{\text{O}}_{\text{3}}}\)?
A. 4
B. \(6.02 \times {10^{23}}\)
C. \(1.20 \times {10^{24}}\)
D. \(2.41 \times {10^{24}}\)
Which statements about solutions are correct?
I. A solute dissolves in a solvent to form a solution.
II. A solution is a homogeneous mixture of two or more substances.
III. Concentrations of solutions can be expressed in \({\text{g}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
The equation for the reduction of iron(III) oxide is:
\[{\text{F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{3CO(g)}} \to {\text{2Fe(s)}} + {\text{3C}}{{\text{O}}_2}{\text{(g)}}\]
What mass of carbon dioxide, in g, is produced by the complete reduction of 80 g of iron(III) oxide?
A. 44
B. 66
C. 88
D. 132
What is the number of ions in 0.20 mol of \({{\text{(N}}{{\text{H}}_{\text{4}}}{\text{)}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\)?
A. \(8.0 \times {10^{ - 1}}\)
B. \(1.2 \times {10^{23}}\)
C. \(4.8 \times {10^{23}}\)
D. \(2.4 \times {10^{24}}\)
A sample of element X contains 69% of 63X and 31% of 65X. What is the relative atomic mass of X in this sample?
A. 63.0
B. 63.6
C. 65.0
D. 69.0
What is the concentration of NaCl, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), when \({\text{10.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaCl solution is added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.600 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaCl solution?
A. 0.450
B. 0.300
C. 0.500
D. 0.800
What is the whole number ratio of the coefficients of ammonia to oxygen when the following equation is balanced correctly?
___\({\text{N}}{{\text{H}}_3}{\text{(g)}} + \)___\({{\text{O}}_2}{\text{(g)}} \to \) ___\({\text{NO(g)}} + \)___\({{\text{H}}_2}{\text{O(l)}}\)
A. 1 : 2
B. 2 : 1
C. 4 : 5
D. 5 : 4
What is the coefficient for \({{\text{O}}_{\text{2}}}{\text{(g)}}\) when the equation for the combustion of 1 mole of pentane is balanced?
\({{\text{C}}_5}{{\text{H}}_{12}}{\text{(g)}} + \) _ \({{\text{O}}_2}{\text{(g)}}\) _ \({\text{C}}{{\text{O}}_2}{\text{(g)}}\) _ \({{\text{H}}_2}{\text{O(g)}}\)
A. 5
B. 6
C. 8
D. 16
What is the pressure, in Pa, in a \({\text{100 c}}{{\text{m}}^{\text{3}}}\)container containing 1.8 g of steam at a temperature of 727 °C? (\(R = 8.31{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\))
A. \(\frac{{{\text{1.8}} \times {\text{8.31}} \times {\text{727}}}}{{{\text{18}} \times {\text{100}}}}\)
B. \(\frac{{{\text{18}} \times {\text{100}}}}{{{\text{1.8}} \times {\text{8.31}} \times {\text{727}}}}\)
C. \(\frac{{{\text{1.8}} \times {\text{8.31}} \times {\text{1000}}}}{{{\text{18}} \times {\text{1}}{{\text{0}}^{ - 4}}}}\)
D. \(\frac{{{\text{1.8}} \times {\text{8.31}}}}{{{\text{1.8}} \times {\text{1}}{{\text{0}}^{ - 4}} \times {\text{1000}}}}\)
The relative molecular mass of a gas is 56 and its empirical formula is \({\text{C}}{{\text{H}}_{\text{2}}}\). What is the molecular formula of the gas?
A. \({\text{C}}{{\text{H}}_{\text{2}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\)
D. \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\)
What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of a substance if 0.30 mol of the substance has a mass of 18 g?
A. 5.4
B. 6.0
C. 30
D. 60
1.7 g of \({\rm{NaN}}{{\rm{O}}_3}({M_r} = 85)\) is dissolved in water to prepare \({\text{0.20 d}}{{\text{m}}^{\text{3}}}\) of solution. What is the concentration of the resulting solution in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)?
A. 0.01
B. 0.1
C. 0.2
D. 1.0
When sodium bromate(V), \({\text{NaBr}}{{\text{O}}_{\text{3}}}\), is heated, it reacts according to the equation below.
\[{\text{2NaBr}}{{\text{O}}_{\text{3}}}{\text{(s)}} \to {\text{2NaBr(s)}} + {\text{3}}{{\text{O}}_{\text{2}}}{\text{(g)}}\]
What amount, in mol, of \({\text{NaBr}}{{\text{O}}_{\text{3}}}\) produces \({\text{2.4 d}}{{\text{m}}^{\text{3}}}\) of oxygen gas, measured at room temperature and pressure? (Molar volume of gas \( = {\text{24 d}}{{\text{m}}^{\text{3}}}{\text{mo}}{{\text{l}}^{ - 1}}\) at room temperature and pressure.)
A. 0.017
B. 0.067
C. 0.10
D. 0.15
Which contains the largest number of ions?
A. 1 mol of \({\text{A}}{{\text{l}}_{\text{2}}}{({\text{S}}{{\text{O}}_{\text{4}}})_{\text{3}}}\)
B. 1 mol of \({\text{M}}{{\text{g}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}\)
C. 2 mol of \({{\text{K}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\)
D. 3 mol of \({\text{NaN}}{{\text{O}}_{\text{3}}}\)
What is the value of x when 32.2 g of Na2SO4•xH2O are heated leaving 14.2 g of anhydrous Na2SO4? Mr(H2O) = 18; Mr(Na2SO4) = 142.
Na2SO4•xH2O (s) → Na2SO4 (s) + xH2O (g)
A. 0.1
B. 1
C. 5
D. 10
\({\text{5 d}}{{\text{m}}^{\text{3}}}\) of carbon monoxide, CO(g), and \({\text{2 d}}{{\text{m}}^{\text{3}}}\) of oxygen, \({{\text{O}}_{\text{2}}}{\text{(g)}}\), at the same temperature and pressure are mixed together. Assuming complete reaction according to the equation given, what is the maximum volume of carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}}\), in \({\text{d}}{{\text{m}}^{\text{3}}}\), that can be formed?
\[{\text{2CO(g)}} + {{\text{O}}_2}({\text{g)}} \to {\text{2C}}{{\text{O}}_2}({\text{g)}}\]
A. 3
B. 4
C. 5
D. 7
What will be the concentration of sulfate ions in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) when 0.20 mol of \({\text{KAl(S}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{2}}}\) is dissolved in water to give \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of aqueous solution?
A. 0.2
B. 1.0
C. 2.0
D. 4.0
On analysis, a compound with molar mass \({\text{60 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) was found to contain 12 g of carbon, 2 g of hydrogen and 16 g of oxygen. What is the molecular formula of the compound?
A. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\)
B. \({\text{C}}{{\text{H}}_{\text{4}}}{\text{O}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{O}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}\)
What is the sum of the coefficients for the equation when balanced using the smallest possible whole numbers?
__ \({{\text{N}}_2}{{\text{H}}_4}({\text{g)}} + \) __ \({{\text{O}}_2}{\text{(g)}} \to \) __ \({\text{N}}{{\text{O}}_2}{\text{(g)}} + \) __ \({{\text{H}}_2}{\text{O(g)}}\)
A. 5
B. 6
C. 7
D. 8
What is the sum of the coefficients when the following equation is balanced using the smallest whole numbers?
__C6H12O6 (aq) → __C2H5OH (aq) + __CO2 (g)
A. 4
B. 5
C. 9
D. 10
What is the sum of the coefficients when the equation is balanced with whole numbers?
—C8H18(g) + —O2(g) → —CO(g) + —H2O(l)
A. 26.5
B. 30
C. 53
D. 61
What is the sum of the coefficients when the following equation is balanced using whole numbers?
___ \({\text{F}}{{\text{e}}_2}{{\text{O}}_3}{\text{(s)}} + \) ___ \({\text{CO(g)}} \to \) ___ \({\text{Fe(s)}} + \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}}\)
A. 5
B. 6
C. 8
D. 9
Why do gases deviate from the ideal gas law at high pressures?
A. Molecules have finite volume.
B. Cohesive forces increase the volume from the ideal.
C. Increasing pressure increases the temperature of the gas.
D. Collisions between molecules occur more frequently as pressure increases.
What volume, in \({{\text{m}}^{\text{3}}}\), is occupied by 2.00 mol of gas at 27 °C and 2.00 atm pressure?
Assume: \({\text{1.00 atm}} = 1.01 \times {10^5}{\text{ Pa}}\) and \(R = 8{\text{.}}31{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\).
A. \(\frac{{8.31 \times 27}}{{1.01 \times {{10}^5}}}\)
B. \(\frac{{2.00 \times 8.31 \times 27}}{{1.01 \times {{10}^5}}}\)
C. \(\frac{{2.00 \times 8.31 \times 300}}{{2.00 \times 1.01 \times {{10}^5}}}\)
D. \(\frac{{2.00 \times 8.31 \times 300}}{{1.01 \times {{10}^5}}}\)
How many moles of oxygen atoms are there in 0.500 mol of hydrated iron(II) ammonium sulfate, (NH4)2Fe(SO4)2•6H2O(s)?
A. 4.00
B. 7.00
C. 8.00
D. 14.00
A fixed mass of gas has a certain volume at a temperature of 50 °C. What temperature is required to double its volume while keeping the pressure constant?
A. 100 K
B. 323 K
C. 373 K
D. 646 K
What is the sum of the coefficients when the equation is balanced with the lowest whole number ratio?
__Na2S2O3(aq) + __HCl(aq) → __S(s) + __SO2(g) + __NaCl(aq) + __H2O(l)
A. 6
B. 7
C. 8
D. 9
What is the number of atoms of oxygen in 2.0 mol of hydrated sodium carbonate, Na2CO3•10H2O? Avogadro’s constant, L or NA: 6.02 × 1023 mol–1
A. 6
B. 26
C. 3.6 × 1024
D. 1.6 × 1025
5.0 cm3 of 2.00 mol\(\,\)dm–3 sodium carbonate solution, Na2CO3(aq), was added to a volumetric flask and the volume was made up to 500 cm3 with water. What is the concentration, in mol\(\,\)dm–3, of the solution?
A. 0.0050
B. 0.0040
C. 0.020
D. 0.010
What is the molecular formula of a hydrocarbon containing 84.6% carbon by mass with a molar mass of 142.3 g mol−1?
A. C20H44
B. C11H10
C. C10H22
D. C5H11
What is the expression for the volume of hydrogen gas, in dm3, produced at STP when 0.30 g of magnesium reacts with excess hydrochloric acid solution?
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
Molar volume of an ideal gas at STP = 22.7 dm3\(\,\)mol−1
A. \(\frac{{0.30 \times 2 \times 22.7}}{{24.31}}\)
B. \(\frac{{0.30 \times 22.7}}{{24.31}}\)
C. \(\frac{{0.30 \times 24.31}}{{22.7}}\)
D. \(\frac{{0.30 \times 22.7}}{{24.31 \times 2}}\)
How many grams of sodium azide, NaN3, are needed to produce 68.1 dm3 of N2 (g) at STP?
Molar volume at STP = 22.7 dm3 mol–1; Mr(NaN3) = 65.0
2NaN3 (s) → 3N2 (g) + 2Na (s)
A. 32.5
B. 65.0
C. 130.0
D. 195.0
Which compound has the greatest percentage by mass of nitrogen atoms?
A. N2H4
B. NH3
C. N2O4
D. NaNO3
What is the coefficient of \({\rm{F}}{{\rm{e}}_3}{{\rm{O}}_4}\) when the following equation is balanced using the lowest whole numbers?
__ \({\text{Al(s)}} + \) __ \({\text{F}}{{\text{e}}_3}{{\text{O}}_4}({\text{s)}} \to \) __ \({\text{A}}{{\text{l}}_2}{{\text{O}}_3}({\text{s)}} + \) __ \({\text{Fe(s)}}\)
A. 2
B. 3
C. 4
D. 5
What is the maximum volume, in dm3, of CO2(g) produced when 1.00 g of CaCO3(s) reacts with 20.0 cm3 of 2.00 mol\(\,\)dm–3 HCl(aq)?
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
Molar volume of gas = 22.7 dm3\(\,\)mol–1; Mr(CaCO3) = 100.00
A. \(\frac{1}{2} \times \frac{{20.0 \times 2.0}}{{1000}} \times 22.7\)
B. \(\frac{{20.0 \times 2.0}}{{1000}} \times 22.7\)
C. \(\frac{{1.0}}{{100.00}} \times 22.7\)
D. \(\frac{{1.0}}{{100.00}} \times 2 \times 22.7\)
The molar mass of a compound is approximately \({\text{56 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Which formula is possible for this compound?
A. \({\text{NaN}}{{\text{O}}_{\text{3}}}\)
B. AgOH
C. MgO
D. KOH
The volume of an ideal gas at 27.0 °C is increased from \({\text{3.00 d}}{{\text{m}}^{\text{3}}}\) to \({\text{6.00 d}}{{\text{m}}^{\text{3}}}\). At what temperature, in °C, will the gas have the original pressure?
A. 13.5
B. 54.0
C. 327
D. 600
Which non-metal forms an oxide XO2 with a relative molecular mass of 60?
A. C
B. N
C. Si
D. S
B. HgCl2(s)→HgCl2(g)
C. I2(g)→I2(s)
D. CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
What is the percentage yield when 7 g of ethene produces 6 g of ethanol?
Mr(ethene) = 28 and Mr(ethanol) = 46
C2H4(g) + H2O(g) → C2H5OH(g)
A. \(\frac{{6 \times 7 \times 100}}{{28 \times 46}}\)
B. \(\frac{{6 \times 46 \times 100}}{{7 \times 28}}\)
C. \(\frac{{6 \times 28}}{{7 \times 46 \times 100}}\)
D. \(\frac{{6 \times 28 \times 100}}{{7 \times 46}}\)
What is the volume, in cm3, of the final solution if 100 cm3 of a solution containing 1.42 g of sodium sulfate, Na2SO4, is diluted to the concentration of 0.020 mol dm–3?
Mr(Na2SO4) = 142
A. 50
B. 400
C. 500
D. 600
The complete combustion of 15.0cm3 of a gaseous hydrocarbon X produces 60.0 cm3 of carbon dioxide gas and 75.0 cm3 of water vapour. What is the molecular formula of X? (All volumes are measured at the same temperature and pressure.)
A. C4H6
B. C4H8
C. C4H10
D. C6H10
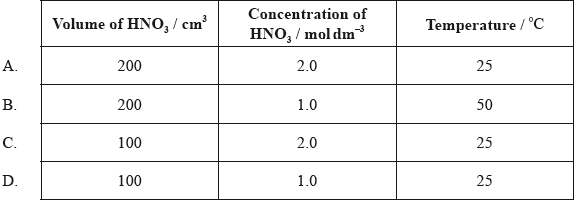
At 25 °C, \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid is added to 5.0 g of magnesium powder. If the experiment is repeated using the same mass of magnesium powder, which conditions will result in the same initial reaction rate?
What is the amount, in moles, of sulfate ions in \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.020 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ FeS}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\)?
A. \(2.0 \times {10^{ - 3}}\)
B. \(2.0 \times {10^{ - 2}}\)
C. \(2.0 \times {10^{ - 1}}\)
D. 2.0
Which volume, in cm3, of 0.20 mol dm-3 NaOH (aq) is needed to neutralize 0.050 mol of H2S(g)?
H2S(g) + 2NaOH(aq) → Na2S(aq) + 2H2O(l)
A. 0.25
B. 0.50
C. 250
D. 500
\({\text{3.0 d}}{{\text{m}}^{\text{3}}}\) of ethyne, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\), is mixed with \({\text{3.0 d}}{{\text{m}}^{\text{3}}}\) of hydrogen and ignited. The equation for the reaction that occurs is shown below.
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}}\]
Assuming the reaction goes to completion and all gas volumes are measured at the same temperature and pressure, what volume of ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), in \({\text{d}}{{\text{m}}^{\text{3}}}\), is formed?
A. 1.5
B. 2.0
C. 3.0
D. 6.0
Chloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\), reacts with oxygen according to the equation below.
\[2{{\text{C}}_2}{{\text{H}}_3}{\text{Cl(g)}} + 5{{\text{O}}_2}({\text{g)}} \to {\text{4C}}{{\text{O}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(g)}} + 2{\text{HCl(g)}}\]
What is the amount, in mol, of \({{\text{H}}_{\text{2}}}{\text{O}}\) produced when 10.0 mol of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\) and 10.0 mol of \({{\text{O}}_{\text{2}}}\) are mixed together, and the above reaction goes to completion?
A. 4.00
B. 8.00
C. 10.0
D. 20.0
How many atoms of nitrogen are there in 0.50 mol of (NH4)2CO3?
A. 1
B. 2
C. 3.01 × 1023
D. 6.02 × 1023
Which is a homogeneous mixture?
A. Oil and water
B. Sand and water
C. Ethanol and water
D. Chalk and sand
In a reaction that occurs in 50 g of aqueous solution, the temperature of the reaction mixture increases by 20 °C. If 0.10 mol of the limiting reagent is consumed, what is the enthalpy change (in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) for the reaction? Assume the specific heat capacity of the solution \( = 4.2{\rm{k}}{{\rm{J}}^{ - 1}}{{\rm{K}}^{ - 1}}\).
A. \( - 0.10 \times 50 \times 4.2 \times 20\)
B. \( - 0.10 \times 0.050 \times 4.2 \times 20\)
C. \(\frac{{ - 50 \times 4{\text{.}}2 \times 20}}{{0{\text{.}}10}}\)
D. \(\frac{{ - 0{\text{.}}050 \times 4{\text{.}}2 \times 20}}{{0{\text{.}}10}}\)
Which factors affect the molar volume of an ideal gas?
\(\begin{array}{*{20}{l}} {{\text{I.}}}&{{\text{Pressure}}} \\ {{\text{II.}}}&{{\text{Temperature}}} \\ {{\text{III.}}}&{{\text{Empirical formula}}} \end{array}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which of the following is consistent with Avogadro’s law?
A. \(\frac{P}{T} = \) constant (\(V\), \(n\) constant)
B. \(\frac{V}{T} = \) constant (\(P\), \(n\) constant)
C. \(Vn = \) constant (\(P\), \(T\) constant)
D. \(\frac{V}{n} = \) constant (\(P\), \(T\) constant)
Which statements about mixtures are correct?
\(\begin{array}{*{20}{l}} {{\text{I.}}}&{{\text{The components may be elements or compounds.}}} \\ {{\text{II.}}}&{{\text{All components must be in the same phase.}}} \\ {{\text{III.}}}&{{\text{The components retain their individual properties.}}} \end{array}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the sum of all coefficients when the following equation is balanced using the smallest possible whole numbers?
__ \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}} + \) __ \({{\text{O}}_{\text{2}}} \to \) __ \({\text{C}}{{\text{O}}_{\text{2}}} + \) __ \({{\text{H}}_{\text{2}}}{\text{O}}\)
A. 5
B. 7
C. 11
D. 13
Some sodium chloride is dissolved in water. Which term describes the role of sodium chloride in this process?
A. Solute
B. Solvent
C. Solution
D. Saturated
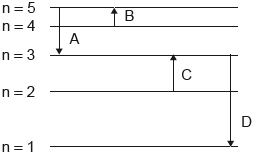
Which electron transition emits energy of the longest wavelength?
What volume of sulfur trioxide, in cm3, can be prepared using \({\text{40 c}}{{\text{m}}^{\text{3}}}\) sulfur dioxide and \({\text{20 c}}{{\text{m}}^{\text{3}}}\) oxygen gas by the following reaction? Assume all volumes are measured at the same temperature and pressure.
\[{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2S}}{{\text{O}}_3}{\text{(g)}}\]
A. 20
B. 40
C. 60
D. 80
7.102 g of \({\text{N}}{{\text{a}}_2}{\text{S}}{{\text{O}}_4}{\text{ (}}M = 142.04{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\) is dissolved in water to prepare \({\text{0.5000 d}}{{\text{m}}^{\text{3}}}\) of solution. What is the concentration of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)?
A. \(2.500 \times {10^{ - 2}}\)
B. \(1.000 \times {10^{ - 1}}\)
C. \(1.000 \times 10\)
D. \(1.000 \times {10^2}\)
The structural formula of a dioxin is shown below.
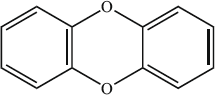
What is its empirical formula?
A. \({{\text{C}}_{\text{6}}}{\text{O}}\)
B. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{O}}\)
C. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{O}}\)
D. \({{\text{C}}_{{\text{12}}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\)
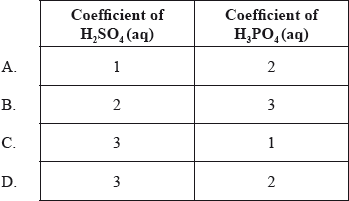
What are the coefficients of \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) and \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\) when the following equation is balanced using the smallest possible whole numbers?
___ \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}{\text{(s)}} + \) ___ \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to \) ___ \({\text{CaS}}{{\text{O}}_4}{\text{(s)}} + \) ___ \({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\)
Which is the best description of relative atomic mass, Ar?
A. The number of neutrons and protons present in the nucleus of an atom
B. The average number of neutrons and protons in all isotopes of an element
C. The weighted mean mass of naturally occurring isotopes of an element compared to the mass of an atom of carbon-12
D. The weighted mean mass of naturally occurring isotopes of an element compared to 1/12th of the mass of an atom of carbon-12
Aluminium carbide reacts with water according to the equation below. What is the sum of all the coefficients when the equation is balanced?
___ \({\text{A}}{{\text{l}}_{\text{4}}}{{\text{C}}_{\text{3}}}{\text{(s)}} + \) ___ \({{\text{H}}_{\text{2}}}{\text{O(l)}} \to \) ___ \({\text{Al(OH}}{{\text{)}}_{\text{3}}}{\text{(s)}} + \) ___ \({\text{C}}{{\text{H}}_{\text{4}}}{\text{(g)}}\)
A. 13
B. 14
C. 19
D. 20
Which represents an empirical formula?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
C. \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}\)
D. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\)
What is the molar mass, in \({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), of washing soda crystals, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}} \bullet {\text{10}}{{\text{H}}_{\text{2}}}{\text{O}}\)?
A. 105.99
B. 124.00
C. 263.15
D. 286.19
When \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of a hydrocarbon, \({{\text{C}}_{\text{x}}}{{\text{H}}_{\text{y}}}\), was burned in excess oxygen, \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of carbon dioxide and \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of steam were produced (all volumes were measured under the same conditions). What is the molecular formula of the hydrocarbon?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\)
D. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
What mass of carbon dioxide, CO2(g), in g, is produced when 5.0 g of calcium carbonate, CaCO3(s),reacts completely with hydrochloric acid, HCl(aq)?
\[{\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {\text{2HCl}}({\text{aq)}} \to {\text{CaC}}{{\text{l}}_2}({\text{aq)}}+{{\text{H}}_2}{\text{O}}({\text{l)+C}}{{\text{O}}_2}({\text{g)}}\]
A. 0.050
B. 2.2
C. 4.4
D. 5.0
Which compound has the highest percentage of carbon by mass?
A. \({\text{C}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
D. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}\)
A gas with a molar mass (\(M\)) of \({\text{44 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) occupies a volume of \(2.00 \times {10^3}{\text{ c}}{{\text{m}}^3}\) at a pressure of \(1.01 \times {10^5}{\text{ Pa}}\) and a temperature of 25 °C. Which expression is correct for the calculation of the mass of the gas, in g? \((R = 8.31{\text{ J}}\,{{\text{K}}^{ - 1}}\,{\text{mo}}{{\text{l}}^{ - 1}})\)
A. \(\frac{{44 \times 1.01 \times {{10}^5} \times 2.00 \times {{10}^{ - 3}}}}{{8{\text{.}}31 \times 298}}\)
B. \(\frac{{44 \times 1{\text{.}}01 \times {{10}^5} \times 2.00 \times {{10}^3}}}{{8{\text{.}}31 \times 25}}\)
C. \(\frac{{1{\text{.}}01 \times {{10}^5} \times 2.00 \times {{10}^{ - 3}}}}{{44 \times 8{\text{.}}31 \times 298}}\)
D. \(\frac{{44 \times 1{\text{.}}01 \times {{10}^5} \times 2.00 \times {{10}^3}}}{{8{\text{.}}31 \times 298}}\)
Which sample contains the largest amount, in mol, of oxygen atoms?
A. 0.20 mol \({{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}\)
B. 0.30 mol \({{\text{O}}_{\text{3}}}\)
C. 0.40 mol \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
D. 0.80 mol \({{\text{H}}_{\text{2}}}{\text{O}}\)
For which compounds is the empirical formula the same as the molecular formula?
I. Methane
II. Ethene
III. Ethanol
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Equal masses of the metals Na, Mg, Ca and Ag are added to separate samples of excess HCl (aq). Which metal produces the greatest total volume of H2(g)?
A. Na
B. Mg
C. Ca
D. Ag
What is the total number of protons and electrons in one mole of hydrogen gas?
A. 2
B. 4
C. \(1.2 \times {10^{24}}\)
D. \(2.4 \times {10^{24}}\)
Which solution contains the biggest amount, in mol, of chloride ions?
A. \({\text{20 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl}}\)
B. \({\text{60 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{MgC}}{{\text{l}}_{\text{2}}}\)
C. \({\text{70 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.30 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{NaCl}}\)
D. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.30 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ClC}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
8.5 g of \({\text{N}}{{\text{H}}_{\text{3}}}\) are dissolved in \({{\text{H}}_{\text{2}}}{\text{O}}\) to prepare a \({\text{500 c}}{{\text{m}}^{\text{3}}}\) solution. Which statements are correct?
I. \({\text{N}}{{\text{H}}_{\text{3}}}\) is the solute and \({{\text{H}}_{\text{2}}}{\text{O}}\) is the solution
II. The concentration of the solution is \({\text{17 g}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
III. \({\text{[N}}{{\text{H}}_3}{\text{]}} = 1.0{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which compound has the empirical formula with the largest mass?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
D. \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\)
Which statements are correct about Avogadro’s constant?
I. It is the number of ions in 12 g of sodium hydride, NaH.
II. It is the number of molecules in \({\text{22.4 d}}{{\text{m}}^{\text{3}}}\) of hydrogen gas at 0 °C and 1 atm.
III. It is the number of atoms in 12 g of \(^{{\text{12}}}{\text{C}}\).
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which molecular formula is also an empirical formula?
A. \({\text{PC}}{{\text{l}}_{\text{3}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)
D. \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\)
At which temperature, in K, assuming constant pressure, is the volume of a fixed mass of gas at 127 °C doubled?
A. 200 K
B. 254 K
C. 400 K
D. 800 K
What is the maximum mass, in g, of magnesium oxide that can be obtained from the reaction of oxygen with 2.4 g of magnesium?
A. 2.4
B. 3.0
C. 4.0
D. 5.6
What is the mass, in g, of one molecule of ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)?
A. \(3.0 \times {10^{ - 23}}\)
B. \(5.0 \times {10^{ - 23}}\)
C. 30
D. \(1.8 \times {10^{25}}\)
What volume of carbon dioxide, CO2(g), in dm3, is produced when 1 dm3 of octane, C8H18(g), undergoes complete combustion?
\[{\text{2}}{{\text{C}}_8}{{\text{H}}_{18}}({\text{g)}} + {\text{25}}{{\text{O}}_2}({\text{g)}} \to {\text{16C}}{{\text{O}}_2}({\text{g)}} + {\text{18}}{{\text{H}}_2}{\text{O}}({\text{g)}}\]
A. 1
B. 4
C. 8
D. 9
Combustion of ethanol takes place according to the following unbalanced equation.
___ \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH(l)}} + \) ___ \({{\text{O}}_{\text{2}}}{\text{(g)}} \to \) ___ \({\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + \) ___ \({{\text{H}}_{\text{2}}}{\text{O(l)}}\)
What is the mole ratio of ethanol to oxygen in the balanced equation?
A. 1:1
B. 2:1
C. 1:3
D. 2:7
What is the sum of all coefficients for the combustion of one mole of propane?
___ \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{(g)}} + \) ___ \({{\text{O}}_{\text{2}}}{\text{(g)}} \to \) ___ \({\text{C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + \) ___ \({{\text{H}}_{\text{2}}}{\text{O(l)}}\)
A. 8
B. 12
C. 13
D. 15
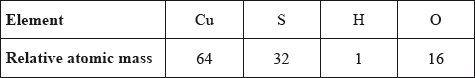
What is the mass, in g, of one mole of hydrated copper(II) sulfate, \({\text{CuS}}{{\text{O}}_{\text{4}}} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}}\), given the following relative atomic mass values?
A. 160
B. 178
C. 186
D. 250
A hydrocarbon contains 85.7 % carbon by mass. What is the empirical formula of the hydrocarbon?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{2}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}\)
\({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of \({\text{BaC}}{{\text{l}}_{\text{2}}}\) is added to \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\). A precipitate of \({\text{BaS}}{{\text{O}}_{\text{4}}}\) is formed according to the equation below.
\[{\text{BaC}}{{\text{l}}_2}{\text{(aq)}} + {\text{N}}{{\text{a}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {\text{BaS}}{{\text{O}}_4}{\text{(s)}} + {\text{2NaCl(aq)}}\]
What is the amount, in mol, of \({\text{BaS}}{{\text{O}}_{\text{4}}}\) produced?
A. 0.0050
B. 0.010
C. 0.050
D. 0.10
0.040 mol of \({{\text{(N}}{{\text{H}}_{\text{4}}}{\text{)}}_{\text{2}}}{\text{Ni (S}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{2}}} \bullet {\text{6}}{{\text{H}}_{\text{2}}}{\text{O}}\) is dissolved in water to give \({\text{200 c}}{{\text{m}}^{\text{3}}}\) of aqueous solution. What is the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of ammonium ions?
A. 0.00040
B. 0.0080
C. 0.20
D. 0.40
What is the pressure, in Pa, if 3 mol of gas occupies \({\text{500 c}}{{\text{m}}^{\text{3}}}\) at 25 °C?
Given: \(R = 8.31{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\)
\({10^{ - 3{\text{ }}}}{{\text{m}}^3} = {10^3}{\text{ c}}{{\text{m}}^3}\)
A. \(\frac{{3 \times 8.31 \times 298}}{{500}}\)
B. \(\frac{{3 \times 8.31 \times 25}}{{0.0005}}\)
C. \(\frac{{3 \times 8.31 \times 25}}{{500}}\)
D. \(\frac{{3 \times 8.31 \times 298}}{{0.0005}}\)
Which volumes of gases at standard temperature and pressure have the same mass as \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({{\text{O}}_{\text{2}}}\)?
I. \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\)
II. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{C}}{{\text{H}}_{\text{4}}}\)
III. \({\text{100 c}}{{\text{m}}^{\text{3}}}\) of \({\text{Si}}{{\text{H}}_{\text{4}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
For which compound is the empirical formula the same as the molecular formula?
Ar(H)=1; Ar(C)=12; Ar(O)=16
5.0mol of Fe2O3(s) and 6.0mol of CO(g) react according to the equation below. What is the limiting reactant and how many moles of the excess reactant remain unreacted?
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)