
HL Paper 2
Magnesium, a reactive metal found in many common minerals, is also an essential nutrient for both plants and animals.
Successive ionization energies of magnesium are given in the table below.

Magnesium metal is mainly used as a component in lightweight alloys, particularly in combination with aluminium and titanium.
Magnesium is usually produced by the electrolysis of molten magnesium chloride.
Define the term first ionization energy.
(i) Explain why the second ionization energy is greater than the first ionization energy.
(ii) Explain why the third ionization energy is much greater than the second ionization energy.
Although magnesium is usually found as \({\text{M}}{{\text{g}}^{2 + }}\) in its compounds, it is possible to use the Born-Haber cycle to investigate the possibility of \({\text{M}}{{\text{g}}^ + }\) being able to form stable compounds.
Use the ionization energy data from part (b), along with the other data provided below, to determine the enthalpy change of formation of MgCl(s). Assume that, because \({\text{M}}{{\text{g}}^ + }\) would be similar in size to \({\text{N}}{{\text{a}}^ + }\), MgCl would have a similar lattice enthalpy to NaCl.
Enthalpy of atomization of Mg \( + 146{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Bond enthalpy in \({\text{C}}{{\text{l}}_{\text{2}}}\) \( + 243{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Electron affinity of Cl \( + 349{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Lattice enthalpy of NaCl \( + 790{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
Consider the lattice enthalpies of \({\text{Mg}}{{\text{F}}_{\text{2}}}\), \({\text{MgC}}{{\text{l}}_2}\) and \({\text{CaC}}{{\text{l}}_{\text{2}}}\). List these from the most endothermic to the least endothermic and explain your order.
\({\text{Most endothermic}} \to {\text{Least endothermic}}\)
Magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), is only sparingly soluble in water and the equilibrium below exists when excess solid is in contact with a saturated solution.
\[{\text{Mg(OH}}{{\text{)}}_2}{\text{(s)}} \rightleftharpoons {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}}\]
Outline how the solubility of magnesium hydroxide will vary with pH.
(i) Describe the bonding present in magnesium metal.
(ii) Suggest why magnesium is harder than sodium.
(iii) Outline why alloys are generally less malleable than their component metals.
(i) Draw a labelled diagram of a suitable apparatus for the electrolysis.
(ii) State equations for the reactions that take place at the electrodes.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iii) When dilute aqueous magnesium chloride is used as the electrolyte, the reactions at both electrodes are different. State equations for the reactions that occur in aqueous solution.
Negative electrode (cathode) reaction:
Positive electrode (anode) reaction:
(iv) Outline why magnesium metal is not produced in the electrolysis of aqueous magnesium chloride.
Buta-1,3-diene can be hydrogenated to produce butane, according to the reaction below.
\[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{(g)}}\]
State the conditions necessary for this reaction.
Determine the standard enthalpy change of reaction, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K for the hydrogenation reaction, using Table 11 of the Data Booklet.
Calculate the standard free energy change, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K for the hydrogenation reaction, using Table 11 of the Data Booklet.
(i) Determine the standard entropy change of the reaction, \(\Delta {S^\Theta }\), at 298 K, in \({\text{kJ}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using your answers from (b) and (c).
(ii) Explain why the standard entropy change for the hydrogenation of buta-1,3-diene has a negative sign.
(iii) Predict whether the hydrogenation reaction becomes more or less spontaneous as the temperature increases.
(iv) Determine the temperature, in K, at which the spontaneity changes.
(v) Determine the standard entropy, \({S^\Theta }\), for hydrogen in \({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using Table 11 of the Data Booklet and your answer for (d)(i).
The Born-Haber cycle for MgO under standard conditions is shown below.
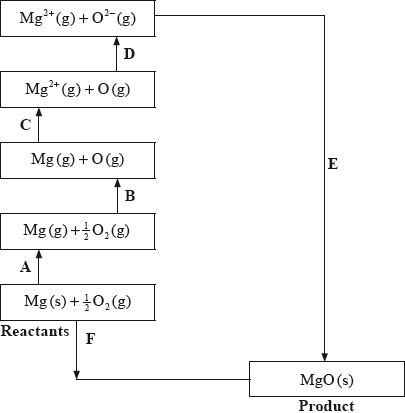
The values are shown in the table below.
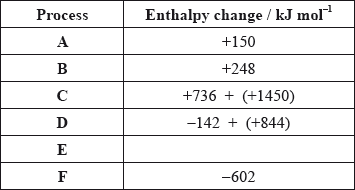
Identify the processes represented by A, B and D in the cycle.
Define the enthalpy change, F.
Determine the value of the enthalpy change, E.
Define the enthalpy change C for the first value. Explain why the second value is significantly larger than the first.
The inter-ionic distance between the ions in NaF is very similar to that between the ions in MgO. Suggest with a reason, which compound has the higher lattice enthalpy value.
The standard enthalpy change of three combustion reactions is given below in kJ.
\[\begin{array}{*{20}{l}} {{\text{2}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{7}}{{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{4C}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}}}&{\Delta {H^\Theta } = - 3120} \\ {{\text{2}}{{\text{H}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{2}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{572}}} \\ {{{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - {\text{1411}}} \end{array}\]
Based on the above information, calculate the standard change in enthalpy, \(\Delta {H^\Theta }\), for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Predict, stating a reason, whether the sign of \({\Delta {S^\Theta }}\) for the above reaction would be positive or negative.
Discuss why the above reaction is non-spontaneous at low temperature but becomes spontaneous at high temperatures.
Using bond enthalpy values, calculate \(\Delta {H^\Theta }\) for the following reaction.
\[{{\text{C}}_2}{{\text{H}}_6}({\text{g)}} \to {{\text{C}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{H}}_2}{\text{(g)}}\]
Suggest with a reason, why the values obtained in parts (b) (i) and (b) (iv) are different.
Lattice enthalpies can be determined experimentally using a Born–Haber cycle and theoretically using calculations based on electrostatic principles.
The experimental lattice enthalpies of the chlorides of lithium, LiCl, sodium, NaCl, potassium, KCl, and rubidium, RbCl, are given in Table 13 of the Data Booklet. Explain the trend in the values.
Explain why magnesium chloride, \({\text{MgC}}{{\text{l}}_{\text{2}}}\), has a much greater lattice enthalpy than sodium chloride, NaCl.
(i) Identify the process labelled a on the Born–Haber cycle for the determination of the standard enthalpy of formation of lithium fluoride, LiF.
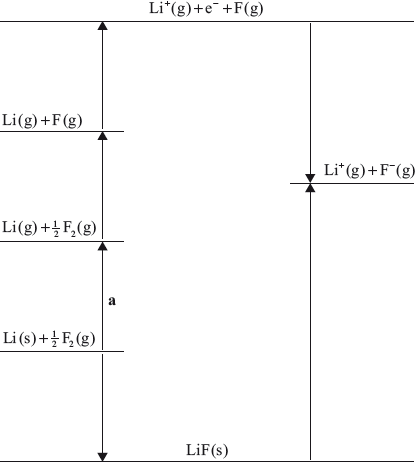
(ii) The enthalpy change for process a is \( + 159{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the standard enthalpy of formation of lithium fluoride, LiF, using this and other values from the Data Booklet.
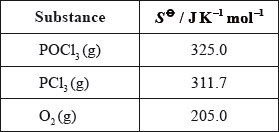
Phosphoryl chloride, \({\text{POC}}{{\text{l}}_{\text{3}}}\), is a dehydrating agent.
\({\text{POC}}{{\text{l}}_{\text{3}}}\left( {\text{g}} \right)\) decomposes according to the following equation.
\[{\text{2POC}}{{\text{l}}_3}{\text{(g)}} \to {\text{2PC}}{{\text{l}}_3}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}}\]
POCl3 can be prepared by the reaction of phosphorus pentachloride, PCl5 , with tetraphosphorus decaoxide, P4O10.
PCl3 and Cl– can act as ligands in transition metal complexes such as Ni(PCl3)4 and [Cr(H2O)3Cl3].
Predict and explain the sign of the entropy change, \(\Delta S\), for this reaction.
Calculate the standard entropy change for the reaction, \(\Delta {S^\Theta }\), in \({\text{J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
Define the term standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \).
Calculate the standard enthalpy change for the reaction, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the data below.
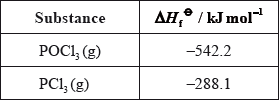
Determine the standard free energy change for the reaction, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), at 298 K.
Deduce the temperature, in K, at which the reaction becomes spontaneous.
Deduce the Lewis (electron dot) structure of POCl3 (with P as the central element) and PCl3 and predict the shape of each molecule, using the valence shell electron pair repulsion theory (VSEPR).
State and explain the Cl–P–Cl bond angle in PCl3.
Deduce the Lewis (electron dot) structure of PCl5.
Predict the shape of this molecule, using the valence shell electron pair repulsion theory (VSEPR).
Identify all the different bond angles in PCl5.
PCl3Br2 has the same molecular shape as PCl5. Draw the three isomers of PCl3Br2 and deduce whether each isomer is polar or non-polar.
Define the term ligand.
Explain why the complex [Cr(H2O)3Cl3] is coloured.
In December 2010, researchers in Sweden announced the synthesis of N,N–dinitronitramide, \({\text{N(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{3}}}\). They speculated that this compound, more commonly called trinitramide, may have significant potential as an environmentally friendly rocket fuel oxidant.
Methanol reacts with trinitramide to form nitrogen, carbon dioxide and water. Deduce the coefficients required to balance the equation for this reaction.
___ \({\text{N(N}}{{\text{O}}_2}{{\text{)}}_3}{\text{(g)}} + \) ___ \({\text{C}}{{\text{H}}_3}{\text{OH(l)}} \to \) ___ \({{\text{N}}_2}{\text{(g)}} + \) ___ \({\text{C}}{{\text{O}}_2}{\text{(g)}} + \) ___ \({{\text{H}}_2}{\text{O(l)}}\)
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), when one mole of trinitramide decomposes to its elements, using bond enthalpy data from Table 10 of the Data Booklet. Assume that all the N–O bonds in this molecule have a bond enthalpy of \({\text{305 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
The entropy change, \(\Delta S\), for the decomposition of trinitramide has been estimated as \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\). Comment on the sign of \(\Delta S\).
Using \( + 700{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) as the value for the entropy change, along with your answer to part (c), calculate \(\Delta G\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for this reaction at 300 K. (If you did not obtain an answer for part (c), then use the value \( - 1000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Explain how changing the temperature will affect whether or not the decomposition of trinitramide is spontaneous.
Outline how the length of the N–N bond in trinitramide compares with the N–N bond in nitrogen gas, \({{\text{N}}_{\text{2}}}\).
Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
Predict, with an explanation, the polarity of the trinitramide molecule.
An example of a homogeneous reversible reaction is the reaction between hydrogen and iodine.
\[{{\text{H}}_2}{\text{(g)}} + {{\text{I}}_2}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}\]
Propene can be hydrogenated in the presence of a nickel catalyst to form propane. Use the data below to answer the questions that follow.

At a temperature just above 700 K it is found that when 1.60 mol of hydrogen and 1.00 mol of iodine are allowed to reach equilibrium in a \({\text{4.00 d}}{{\text{m}}^{\text{3}}}\) flask, the amount of hydrogen iodide formed in the equilibrium mixture is 1.80 mol. Determine the value of the equilibrium constant at this temperature.
Outline why the value for the standard enthalpy change of formation of hydrogen is zero.
Calculate the standard enthalpy change for the hydrogenation of propene.
Calculate the standard entropy change for the hydrogenation of propene.
Determine the value of \(\Delta {G^\Theta }\) for the hydrogenation of propene at 298 K.
At 298 K the hydrogenation of propene is a spontaneous process. Determine the temperature above which propane will spontaneously decompose into propene and hydrogen.
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]

Determine the \(\Delta {S^\Theta }\) for the combustion of methanol.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]
Using the enthalpy of combustion for methanol from Table 12 of the Data Booklet and the \(\Delta {S^\Theta }\) determined in part (d), calculate the standard free energy change for the combustion of methanol.
Explain whether changing the temperature will alter the spontaneity of the reaction.
Enthalpy changes depend on the number and type of bonds broken and formed.
The table lists the standard enthalpies of formation, \(\Delta H_{\text{f}}^\Theta \), for some of the species in the reaction above.
Enthalpy changes depend on the number and type of bonds broken and formed.
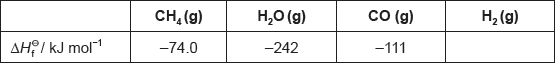
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
Outline why no value is listed for H2(g).
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
The table lists standard entropy, SΘ, values.
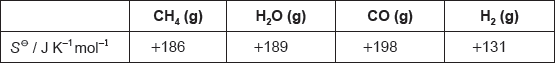
Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
Determine the temperature, in K, above which the reaction becomes spontaneous.
To determine the enthalpy change of combustion of methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\), 0.230 g of methanol was combusted in a spirit burner. The heat released increased the temperature of \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water from 24.5 °C to 45.8 °C.
Methanol can be produced according to the following equation.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \to {\text{C}}{{\text{H}}_3}{\text{OH(l)}}\]
The manufacture of gaseous methanol from CO and \({{\text{H}}_{\text{2}}}\) involves an equilibrium reaction.
\({\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{OH(g)}}\) \(\Delta {H^\Theta } < 0\)
Calculate the standard enthalpy change of this reaction, using the values of enthalpy of combustion in Table 12 of the Data Booklet.
Calculate the standard entropy change for this reaction, \(\Delta {S^\Theta }\), using Table 11 of the Data Booklet and given:
\({S^\Theta }{\text{(CO)}} = 198{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}{\text{ and }}{S^\Theta }{\text{(}}{{\text{H}}_{\text{2}}}{\text{)}} = 131{\text{ J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate, stating units, the standard free energy change for this reaction, \(\Delta {G^\Theta }\), at 298 K.
Predict, with a reason, the effect of an increase in temperature on the spontaneity of this reaction.
1.00 mol of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\) is placed in a closed container of volume \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) until equilibrium is reached with CO and \({{\text{H}}_{\text{2}}}\). At equilibrium 0.492 mol of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\) are present. Calculate \({K_{\text{c}}}\).
Consider the following reaction.
\[{\text{2C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}} + {{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\]
The standard enthalpy change of formation for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}\) at 298 K is \( - 201{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and for \({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is \( - 242{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Using information from Table 11 of the Data Booklet, determine the enthalpy change for this reaction.
The standard entropy for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(g)}}\) at 298 K is \({\text{238 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\), for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) is \({\text{131 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\) and for \({{\text{H}}_{\text{2}}}{\text{O(g)}}\) is \({\text{189 J}}\,{{\text{K}}^{ - 1}}{\text{mo}}{{\text{l}}^{ - 1}}\). Using information from Table 11 of the Data Booklet, determine the entropy change for this reaction.
Calculate the standard change in free energy, at 298 K, for the reaction and deduce whether the reaction is spontaneous or non-spontaneous.
Consider the reaction:
\[{\text{CuS(s)}} + {{\text{H}}_2}{\text{(g)}} \to {\text{Cu(s)}} + {{\text{H}}_2}{\text{S(g)}}\]
Given:
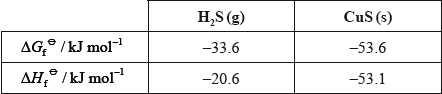
Deduce and explain the sign of the entropy change for the following reaction.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\]
Suggest why the \(\Delta H_{\text{f}}^\Theta \) values for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) and Cu(s) are not given in the table.
Determine the standard enthalpy change at 298 K for the reaction.
Determine the standard free energy change at 298 K for the reaction. Deduce whether or not the reaction is spontaneous at this temperature.
Determine the standard entropy change at 298 K for the reaction.
Estimate the temperature, in K, at which the standard change in free energy equals zero. You should assume that the values of the standard enthalpy and entropy changes are not affected by the change in temperature.
Draw the Lewis structures, state the shape and predict the bond angles for the following species.
Consider the following Born-Haber cycle:
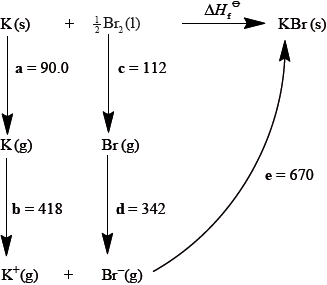
The magnitudes for each of the enthalpy changes (a to e) are given in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) but their signs (+ or –) have been omitted.
\({\text{PC}}{{\text{l}}_{\text{3}}}\)
\({\text{NH}}_2^ - \)
\({\text{Xe}}{{\text{F}}_{\text{4}}}\)
State the names for the enthalpy changes c and d.
Deduce which two of the enthalpy changes a to e have negative signs.
Determine the value for the enthalpy of formation of potassium bromide.
Explain why the quantitative value for the lattice enthalpy of calcium bromide is larger than the value for the lattice enthalpy of potassium bromide.
Compare the formation of a sigma \((\sigma )\) and a pi \((\pi )\) bond between two carbon atoms in a molecule.
Identify how many sigma and pi bonds are present in propene, \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}\).
Deduce all the bond angles present in propene.
Explain how the concept of hybridization can be used to explain the bonding in the triple bond present in propyne.
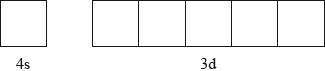
The electron configuration of chromium can be expressed as \({\text{[Ar]4}}{{\text{s}}^{\text{x}}}{\text{3}}{{\text{d}}^{\text{y}}}\).
Hydrogen and nitrogen(II) oxide react according to the following equation.
\[2{{\text{H}}_2}{\text{(g)}} + {\text{2NO(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]
At time = \(t\) seconds, the rate of the reaction is
\[{\text{rate}} = k{\text{[}}{{\text{H}}_2}{\text{(g)][NO(g)}}{{\text{]}}^2}\]
When concentrated hydrochloric acid is added to a solution containing hydrated copper(II) ions, the colour of the solution changes from light blue to green. The equation for the reaction is:
\[{{\text{[Cu(}}{{\text{H}}_2}{\text{O}}{{\text{)}}_6}{\text{]}}^{2 + }}{\text{(aq)}} + {\text{4C}}{{\text{l}}^ - }{\text{(aq)}} \to {{\text{[CuC}}{{\text{l}}_4}{\text{]}}^{2 - }}{\text{(aq)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}}\]
Explain what the square brackets around argon, [Ar], represent.
State the values of \(x\) and \(y\).
Annotate the diagram below showing the 4s and 3d orbitals for a chromium atom using an arrow, 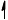 and
and 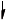 , to represent a spinning electron.
, to represent a spinning electron.
Explain precisely what the square brackets around nitrogen(II) oxide, [NO(g)], represent in this context.
Deduce the units for the rate constant \(k\).
Explain what the square brackets around the copper containing species represent.
Explain why the \({{\text{[Cu(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\) ion is coloured and why the \({{\text{[CuC}}{{\text{l}}_{\text{4}}}{\text{]}}^{2 - }}\) ion has a different colour.
Some words used in chemistry can have a specific meaning which is different to their meaning in everyday English.
State what the term spontaneous means when used in a chemistry context.
Hydrazine, N2H4, is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{l)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(l)}}\]
The reaction between \({{\text{N}}_2}{{\text{H}}_4}({\text{aq)}}\) and \({\text{HCl(aq)}}\) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw the Lewis (electron dot) structure for N2H4 showing all valence electrons.
(ii) State and explain the H–N–H bond angle in hydrazine.
Hydrazine and ethene, C2H4, are hydrides of adjacent elements in the periodic table. The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
(i) The enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of liquid hydrazine is \({\text{50.6 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Use this value, together with data from Table 12 of the Data Booklet, to calculate the enthalpy change for this reaction.
(ii) Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
(iii) Identify the calculation that produces the most accurate value for the enthalpy change for the reaction given and explain your choice.
(iv) Calculate \(\Delta {S^\Theta }\) for the reaction using the data below and comment on its magnitude.
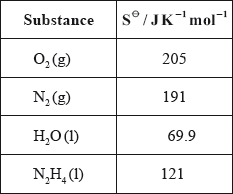
(v) Calculate \(\Delta {G^\Theta }\) for the reaction at 298 K.
(vi) Predict, giving a reason, the spontaneity of the reaction above at both high and low temperatures.
The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and HCl(aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
(iii) Suggest the type of hybridization shown by the nitrogen atoms in \({{\text{N}}_{\text{2}}}{\text{H}}_{\text{6}}^{{\text{2}} + }\).
Ethane-1,2-diol, HOCH2CH2OH, reacts with thionyl chloride, SOCl2, according to the reaction below.
HOCH2CH2OH (l) + 2SOCl2 (l) → ClCH2CH2Cl (l) + 2SO2 (g) + 2HCl (g)
Calculate the standard enthalpy change for this reaction using the following data.
Calculate the standard entropy change for this reaction using the following data.
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Hydrogenation of propene produces propane. Calculate the standard entropy change, ΔS θ, for the hydrogenation of propene.
The standard enthalpy change, ΔH θ, for the hydrogenation of propene is –124.4 kJ mol–1. Predict the temperature above which the hydrogenation reaction is not spontaneous.
Millerite, a nickel sulfide mineral, is an important source of nickel. The first step in extracting nickel is to roast the ore in air.
The reaction for the formation of liquid tetracarbonylnickel is shown below:
\[{\text{Ni(s)}} + 4{\text{CO(g)}} \to {\text{Ni(CO}}{{\text{)}}_4}{\text{(l)}}\]
Formulate an equation for the oxidation of nickel(II) sulfide to nickel(II) oxide.
The nickel obtained from another ore, nickeliferous limonite, is contaminated with iron. Both nickel and iron react with carbon monoxide gas to form gaseous complexes, tetracarbonylnickel, \({\text{Ni(CO}}{{\text{)}}_{\text{4}}}{\text{(g)}}\), and pentacarbonyliron, \({\text{Fe(CO}}{{\text{)}}_{\text{5}}}{\text{(g)}}\). Suggest why the nickel can be separated from the iron successfully using carbon monoxide.
Calculate the standard entropy change, \(\Delta {S^\theta }\), of the reaction, in \({\text{J}}\,{{\text{K}}^{ - 1}}\), using the values given.
Calculate a value for \(\Delta {H^\theta }\) in kJ.
Use your answers to (c)(i) and (c)(ii), to determine the temperature, in °C, at which the decomposition of liquid tetracarbonylnickel to nickel and carbon monoxide becomes favourable.
(If you did not get answers to (c)(i) and (c)(ii), use \( - 500{\text{ J}}\,{{\text{K}}^{ - 1}}\) and \( - 200{\text{ kJ}}\) respectively but these are not the correct answers.)
Suggest why experiments involving tetracarbonylnickel are very hazardous.
This question is about ethene, C2H4, and ethyne, C2H2.
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
Ethyne reacts with chlorine in a similar way to ethene. Formulate equations for the following reactions.
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
One possible Lewis structure for benzene is shown.
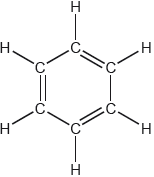
State one piece of physical evidence that this structure is incorrect.
Vanadium has a number of different oxidation states.
Electrode potentials for the reactions of vanadium and other species are shown below.
Determine the oxidation state of vanadium in each of the following species.
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
Identify, from the table, a non-vanadium species that could convert \({\text{VO}}_2^ + {\text{(aq)}}\) to V2+(aq).
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Comment on the spontaneity of this reaction by calculating a value for \(\Delta {G^\theta }\) using the data given in (b) and in section 1 of the data booklet.
The reaction between ethene and steam is used in the industrial production of ethanol.
\[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(g)}} + {{\text{H}}_{\text{2}}}{\text{O(g)}} \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH(g)}}\]
The enthalpy change of the reaction can be calculated either by using average bond enthalpies or by using standard enthalpies of formation.
Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using the average bond enthalpies in Table 10 of the Data Booklet.
(i) Define the term standard enthalpy change of formation.
(ii) Determine the enthalpy change of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), between ethene and steam using the enthalpy change of formation values given below.
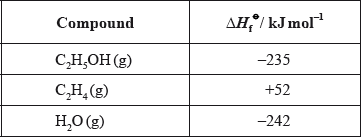
Comment on which of the values obtained in (a) and (b)(ii) is more accurate, giving a reason.
Predict the sign of the entropy change of the reaction, \(\Delta S\), giving a reason.
Tin(II) chloride is a white solid that is commonly used as a reducing agent.
(i) State why you would expect tin(II) chloride to have a similar lattice enthalpy to strontium chloride, using section 9 of the data booklet.
(ii) Calculate the molar enthalpy change when strontium chloride is dissolved in water, using sections 18 and 20 of the data booklet.
(iii) Tin(II) chloride reacts with water to precipitate the insoluble basic chloride, Sn(OH)Cl.
Suggest why tin(II) chloride is usually dissolved in dilute hydrochloric acid.
Tin can also exist in the +4 oxidation state.
Vanadium can be reduced from an oxidation state of +4 to +3 according to the equation:
(i) Calculate the cell potential, EΘ, and the standard free energy, ΔGΘ, change for the reaction between the VO2+ and Sn2+ ions, using sections 1 and 2 of the data booklet.
EΘ:
ΔGΘ:
(ii) Deduce, giving your reason, whether a reaction between Sn2+(aq) and VO2+(aq) would be spontaneous.
Outline, giving the full electron configuration of the vanadium atom, what is meant by the term transition metal.
In an aqueous solution of vanadium(III) chloride, the vanadium exists as [V (H2O)6]3+, [VCl (H2O)5]2+ or [VCl2(H2O)4]+ depending on the concentration of chloride ions in the solution.
(i) Describe how Cl− and H2O bond to the vanadium ion.
(ii) Outline what would happen to the wavelength at which the vanadium complex ions would absorb light as the water molecules are gradually replaced by chloride ions, using section 15 of the data booklet.
Eight successive ionisation energies of vanadium are shown in the graph below:
(i) State the sub-levels from which each of the first four electrons are lost.
First: Second: Third: Fourth:
(ii) Outline why there is an increase in ionization energy from electron 3 to electron 5.
(iii) Explain why there is a large increase in the ionization energy between electrons 5 and 6.
(iv) Vanadium is comprised almost entirely of 51V. State the number of neutrons an atom of 51V has in its nucleus.
Ethanol has many industrial uses.
State an equation for the formation of ethanol from ethene and the necessary reaction conditions.
Equation:
Conditions:
Define the term average bond enthalpy.
Ethanol can be used as a fuel. Determine the enthalpy of combustion of ethanol at 298 K, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), using the values in table 10 of the data booklet, assuming all reactants and products are gaseous.
Students can also measure the enthalpy of combustion of ethanol in the laboratory using calorimetry. Suggest the major source of systematic error in these procedures.
State the equation for the acid-catalysed reaction of ethanol with propanoic acid and state the name of the organic product.
Equation:
Name of the organic product:
A polyester can be formed when ethane-1,2-diol reacts with benzene-1,4-dicarboxylic acid.
Deduce the structure of the repeating unit and state the other product formed.
Repeating unit:
Other product:
State the type of polymerization that occurs.
The standard enthalpy change of combustion, \(\Delta H_{\text{c}}^\Theta \), of propanoic acid is \( - 1527{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine the standard enthalpy change of formation of propanoic acid, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using this information and data from table 12 of the data booklet.
Deduce, giving a reason, the sign of the standard entropy change of the system for the formation of propanoic acid from its elements.
Identify three allotropes of carbon and describe their structures.
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
(i) Calculate ΔHθ, in kJ, for this similar reaction below using \(\Delta H_{\rm{f}}^\theta \) data from section 12 of the data booklet. \(\Delta H_{\rm{f}}^\theta \) of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
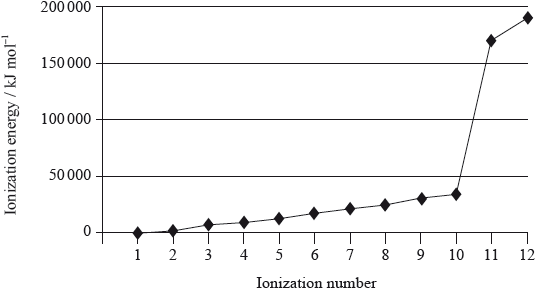
Magnesium is the eighth most abundant element in the earth’s crust. The successive ionization energies of the element are shown below.
Magnesium can be produced from the electrolysis of molten magnesium chloride, MgCl2.
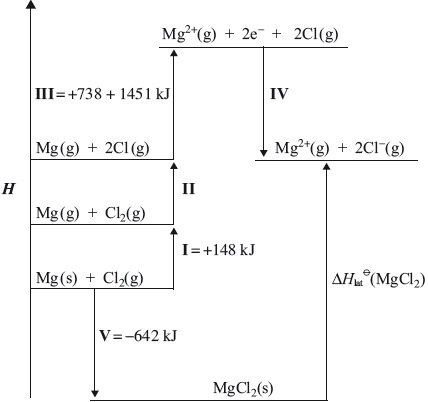
The lattice enthalpy of magnesium chloride can be calculated from the Born-Haber cycle shown below.
(i) Define the term first ionization energy and state the equation for the first ionization of magnesium.
(ii) Explain the general increase in successive ionization energies of the element.
(iii) Explain the large increase between the tenth and eleventh ionization energies.
(i) Explain how molten magnesium chloride conducts an electric current.
(ii) Identify the electrode where oxidation occurs during electrolysis of molten magnesium chloride and state an equation for the half-reaction.
(iii) Explain why magnesium is not formed during the electrolysis of aqueous magnesium chloride solution.
(i) Identify the enthalpy changes labelled by I and V in the cycle.
(ii) Use the ionization energies given in the cycle above and further data from the Data Booklet to calculate a value for the lattice enthalpy of magnesium chloride.
(iii) The theoretically calculated value for the lattice enthalpy of magnesium chloride is +2326 kJ. Explain the difference between the theoretically calculated value and the experimental value.
(iv) The experimental lattice enthalpy of magnesium oxide is given in Table 13 of the Data Booklet. Explain why magnesium oxide has a higher lattice enthalpy than magnesium chloride.
(i) State whether aqueous solutions of magnesium oxide and magnesium chloride are acidic, alkaline or neutral.
(ii) State an equation for the reaction between magnesium oxide and water.
Carbon monoxide reacts with hydrogen to produce methanol.
\[{\text{CO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH(l)}}\]
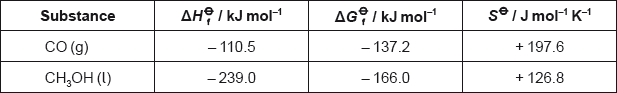
Calculate the standard enthalpy change, \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction.
Calculate the standard free energy change, \(\Delta {G^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - {\text{1}}}}\), for the reaction
\({\text{(}}\Delta G_{\text{f}}^\Theta {\text{(}}{{\text{H}}_{\text{2}}}{\text{)}} = {\text{0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\).
Using the values obtained in parts (a) and (b), calculate the standard entropy change, \(\Delta {S^\Theta }\), in \({\text{J mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for the reaction at 298 K.
Determine the absolute entropy, \({S^\Theta }\), in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ - 1}}{{\text{K}}^{ - 1}}\), for \({{\text{H}}_{\text{2}}}{\text{(g)}}\) at 298 K.
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromomethane reacts with aqueous sodium hydroxide. State the organic product of this reaction.
Explain why the rate of the reaction between iodomethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}\), and NaOH(aq) is faster than the rate of the reaction between \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\) and NaOH(aq).
Bromine can be produced by the electrolysis of molten sodium bromide.
Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Predict the products formed at the electrodes during the electrolysis of concentrated aqueous sodium bromide.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide.
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Define the term standard electrode potential, \({E^\Theta }\).
Draw a labelled diagram for the voltaic cell in which the following reaction occurs.
\[{\text{Mg(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\]
Include in your answer the direction of electron flow and the polarity of the electrodes.
A student measures a voltage of 2.65 V in the voltaic cell formed between magnesium and copper half-cells using a digital voltmeter.
State the random uncertainty of this value, in V, and the number of significant figures in the answer.
Random uncertainty:
Significant figures:
Outline how the student can reduce the random error in her results.
Determine the standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of NaCl(s), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using a Born-Haber cycle and tables 7, 10 and 13 of the data booklet. The standard enthalpy change of atomization (standard enthalpy change of sublimation), \(\Delta H_{{\text{at}}}^\Theta \), of Na(s) is \( + {\text{108 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.