
HL Paper 1
What is the standard half-cell potential of copper if the “zero potential reference electrode” is changed from the standard hydrogen electrode to a standard zinc electrode?

A. –1.1
B. –0.34
C. +0.34
D. +1.1
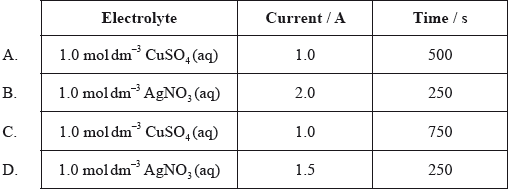
Four electrolytic cells are constructed. Which cell would produce the greatest mass of metal at the negative electrode (cathode)?
What are the major products of electrolysing concentrated aqueous potassium iodide, KI(aq)?

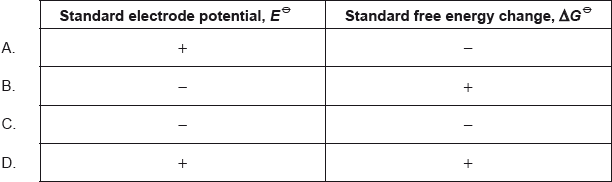
Which signs for both Eθcell and ΔGθ result in a spontaneous redox reaction occurring under standard conditions?
B. NaCl
C. H2SO4
D. AgNO3
An aqueous solution of a metal salt is electrolysed. Which factor will have no effect on the mass of the metal deposited on the negative electrode (cathode), if all other variables remain constant?
A. Size of metal ion
B. Relative atomic mass of metal
C. Current
D. Charge on metal ion
Consider these standard electrode potentials.
\[\begin{array}{*{20}{l}} {{\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Mg(s)}}}&{{E^\Theta } = - 2.36{\text{ V}}} \\ {{\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Zn(s)}}}&{{E^\Theta } = - 0.76{\text{ V}}} \end{array}\]
What is the cell potential for the voltaic cell produced when the two half-cells are connected?
A. –1.60 V
B. +1.60 V
C. –3.12 V
D. +3.12 V
Which are necessary conditions for the standard hydrogen electrode to have an \({E^\Theta }\) of exactly zero?
I. Temperature = 298 K
II. \({\text{[}}{{\text{H}}^ + }{\text{]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
III. \({\text{[}}{{\text{H}}_2}{\text{]}} = 1{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
D. 2z
A number of molten metal chlorides are electrolysed, using the same current for the same length of time. Which metal will be produced in the greatest amount, in mol?
A. Mg
B. Al
C. K
D. Ca
The standard electrode potentials of some half-reactions are given below.
\({\text{S}}{{\text{n}}^{4 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 0.15{\text{ V}}\)
\(\frac{1}{2}{{\text{I}}_2}{\text{(s)}} + {{\text{e}}^ - } \rightleftharpoons {{\text{I}}^ - }{\text{(aq)}}\) \({E^\Theta } = + 0.54{\text{ V}}\)
\({\text{F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 0.77{\text{ V}}\)
Which of the following reactions will occur spontaneously?
A. Iodine reduces \({\text{F}}{{\text{e}}^{3 + }}\) to \({\text{F}}{{\text{e}}^{2 + }}\)
B. Iodine reduces \({\text{S}}{{\text{n}}^{4 + }}\)to \({\text{S}}{{\text{n}}^{2 + }}\)
C. Iodine oxidizes \({\text{F}}{{\text{e}}^{2 + }}\)to \({\text{F}}{{\text{e}}^{3 + }}\)
D. Iodine oxidizes \({\text{S}}{{\text{n}}^{2 + }}\) to \({\text{S}}{{\text{n}}^{4 + }}\)
Which combination would electroplate an object with copper?

Two cells undergoing electrolysis are connected in series.
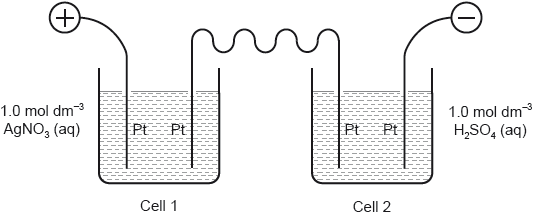
If \(x\) g of silver are deposited in cell 1, what volume of oxygen, in dm3 at STP, is given off in cell 2?
Ar(Ag) = 108; Molar volume of an ideal gas at STP = 22.7 dm3 mol−1
A. \(\frac{x}{{108}} \times \frac{1}{4} \times 22.7\)
B. \(\frac{x}{{108}} \times 4 \times 22.7\)
C. \(\frac{x}{{108}} \times \frac{1}{2} \times 22.7\)
D. \(\frac{x}{{108}} \times 2 \times 22.7\)
The standard electrode potentials for two metals are given below.
\[\begin{array}{*{20}{l}} {{\text{A}}{{\text{l}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Al(s)}}}&{{E^\Theta } = - 1.66{\text{ V}}} \\ {{\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Ni(s)}}}&{{E^\Theta } = - 0.23{\text{ V}}} \end{array}\]
What is the equation and cell potential for the spontaneous reaction that occurs?
\(\begin{array}{*{20}{l}} {{\text{A.}}}&{2{\text{A}}{{\text{l}}^{3 + }}({\text{aq)}} + 3{\text{Ni(s)}} \to {\text{2Al(s)}} + 3{\text{N}}{{\text{i}}^{2 + }}({\text{aq)}}}&{{E^\Theta } = 1.89{\text{ V}}} \\ {{\text{B.}}}&{2{\text{Al(s)}} + 3{\text{N}}{{\text{i}}^{2 + }}({\text{aq)}} \to {\text{2A}}{{\text{l}}^{3 + }}({\text{aq)}} + 3{\text{Ni(s)}}}&{{E^\Theta } = 1.89{\text{ V}}} \\ {{\text{C.}}}&{2{\text{A}}{{\text{l}}^{3 + }}({\text{aq)}} + 3{\text{Ni(s)}} \to {\text{2Al(s)}} + 3{\text{N}}{{\text{i}}^{2 + }}({\text{aq)}}}&{{E^\Theta } = 1.43{\text{ V}}} \\ {{\text{D.}}}&{2{\text{Al(s)}} + 3{\text{N}}{{\text{i}}^{2 + }}({\text{aq)}} \to {\text{2A}}{{\text{l}}^{3 + }}({\text{aq)}} + 3{\text{Ni(s)}}}&{{E^\Theta } = 1.43{\text{ V}}} \end{array}\)
Consider the following standard electrode potentials.
\({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Sn(s)}}\) \({E^\Theta } = - 0.14{\text{ V}}\)
\({{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons \frac{{\text{1}}}{{\text{2}}}{{\text{H}}_{\text{2}}}{\text{(g)}}\) \({E^\Theta } = 0.00{\text{ V}}\)
\({\text{F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 0.77{\text{ V}}\)
Which species will reduce H\(^ + \)(aq) to H\(_2\) (g) under standard conditions?
A. Fe\(^{2 + }\)(aq)
B. Sn\(^{2 + }\)(aq)
C. Sn(s)
D. Fe\(^{3 + }\)(aq)
What happens during the electrolysis of concentrated aqueous potassium chloride?
I. Reduction takes place at the negative electrode (cathode).
II. Hydrogen gas is evolved at the negative electrode (cathode).
III. The pH of the electrolyte increases.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
The standard electrode potentials for three reactions involving copper and copper ions are:
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{C}}{{\text{u}}^ + }{\text{(aq) }}{E^\Theta } = + 0.15{\text{ V}}\)
\({\text{C}}{{\text{u}}^{{\text{2}} + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Cu(s) }}{E^\Theta } = + {\text{0.34 V}}\)
\({\text{C}}{{\text{u}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{Cu(s) }}{E^\Theta } = + {\text{0.52 V}}\)
Which statement is correct?
A. \({\text{C}}{{\text{u}}^{{\text{2}} + }}\) ions are a better oxidizing agent than \({\text{C}}{{\text{u}}^ + }\) ions.
B. Copper metal is a better reducing agent than \({\text{C}}{{\text{u}}^ + }\) ions.
C. \({\text{C}}{{\text{u}}^ + }\) ions will spontaneously form copper metal and \({\text{C}}{{\text{u}}^{{\text{2}} + }}\) ions in solution.
D. Copper metal can be spontaneously oxidized by \({\text{C}}{{\text{u}}^{{\text{2}} + }}\) ions to form \({\text{C}}{{\text{u}}^ + }\) ions.
What are the relative volumes of gas given off at E and F during electrolysis of the two cells in series? Assume all electrodes are inert.
A. 1:1
B. 1:2
C. 2:1
D. 5:2
Two half-cells are connected via a salt bridge to make a voltaic cell. Which statement about this cell is correct?
A. Oxidation occurs at the positive electrode (cathode).
B. It is also known as an electrolytic cell.
C. Ions flow through the salt bridge.
D. It requires a power supply to operate.
Which signs are correct for a spontaneous redox reaction?
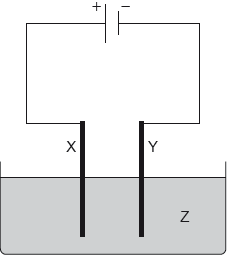
An iron rod is electroplated with silver. Which is a correct condition for this process?
A. The silver electrode is the positive electrode.
B. The iron rod is the positive electrode.
C. The electrolyte is iron(II) sulfate.
D. Oxidation occurs at the negative electrode.
What is the cell potential, in V, of the reaction below?
\[{{\text{I}}_2} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - } \to {\text{2}}{{\text{I}}^ - } + {{\text{S}}_4}{\text{O}}_6^{2 - }\]
\(\frac{1}{2}{{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}}\) \({E^\Theta } = + 0.09{\text{ V}}\)
\({{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{2}}{{\text{I}}^ - }{\text{(aq)}}\) \({E^\Theta } = + 0.54{\text{ V}}\)
A. \( + 0.63\)
B. \( + 0.45\)
C. \( - 0.45\)
D. \( - 0.63\)
What are the products when an aqueous solution of copper(II) sulfate is electrolysed using inert graphite electrodes?
What is the cell potential, in V, for the reaction that occurs when the following two half-cells are connected?
\[\begin{array}{*{20}{l}} {{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Fe(s)}}}&{{E^\Theta } = - 0.44{\text{ V}}} \\ {{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }({\text{aq)}} + 14{{\text{H}}^ + }({\text{aq)}} + 6{{\text{e}}^ - } \rightleftharpoons 2{\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}}&{{E^\Theta } = + 1.33{\text{ V}}} \end{array}\]
A. +0.01
B. +0.89
C. +1.77
D. +2.65
In the electrolysis of aqueous potassium nitrate, KNO3(aq), using inert electrodes, 0.1 mol of a gas was formed at the cathode (negative electrode).
Which is correct?
Consider the standard electrode potentials:
\[{\text{F}}{{\text{e}}^{{\text{2 + }}}}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Fe(s) }} {E^\Theta } = - {\text{0.45 V}}\]
\[\frac{1}{2}{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} + {{\text{e}}^ - } \rightleftharpoons {\text{C}}{{\text{l}}^ - }{\text{(aq) }} {E^\Theta } = {\text{ }} + {\text{1.36 V}}\]
What is the standard cell potential, in V, for the reaction?
\[{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} + {\text{Fe(s)}} \to {\text{2C}}{{\text{l}}^ - }{\text{(aq)}} + {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\]
A. \( + {\text{0.91}}\)
B. \( + {\text{1.81}}\)
C. \( + {\text{2.27}}\)
D. \( + {\text{3.17}}\)
Which statement is correct for electroplating an object with gold?
A. The object must be the negative electrode (cathode).
B. The negative electrode (cathode) must be gold.
C. The object must be the positive electrode (anode).
D. The gold electrode must be pure.
Consider the following standard electrode potentials:
\[\begin{array}{*{20}{l}} {{\text{S}}{{\text{n}}^{4 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}}&{{E^\Theta } = + 0.13{\text{ V}}} \\ {{\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Pb(s)}}}&{{E^\Theta } = - 0.13{\text{ V}}} \end{array}\]
What is the value of the cell potential, in V, for the spontaneous reaction that occurs when the two half-cells are connected together?
A. \( - 0.26\)
B. 0.00
C. \( + 0.13\)
D. \( + 0.26\)
The same quantity of electricity is passed through separate dilute aqueous solutions of sulfuric acid and copper(II) sulfate using platinum electrodes under the same conditions. Which statement is correct?
A. The same volume of oxygen is obtained in both cases.
B. The same volume of hydrogen is obtained in both cases.
C. The amount of copper deposited at the negative electrode in the copper(II) sulfate solution is half the amount of hydrogen gas formed at the negative electrode in the sulfuric acid solution.
D. The pH of both solutions increases as the electrolysis proceeds.
Which statement is correct for the overall reaction in a voltaic cell?
2AgNO3(aq) + Ni(s) → 2Ag(s) + Ni(NO3)2(aq) E θ= +1.06 V
A. Electrons flow from Ag electrode to Ni electrode.
B. Ni is oxidized to Ni2+ at the cathode (negative electrode).
C. Ag+ is reduced to Ag at the anode (positive electrode).
D. Ag has a more positive standard electrode potential value than Ni.
Two electrolytic cells are connected in series and the same current passes through each cell. The first cell contains silver electrodes in silver nitrate solution. The second cell contains copper electrodes in copper(II) sulfate solution. In one experiment 1.00 g of silver is deposited in the first cell. What mass of copper, in g, is deposited in the second cell?
A. \(\frac{{1.00}}{{107.87}}\)
B. \(\frac{{1.00}}{{63.55}}\)
C. \(\frac{{1.00}}{{107.87}} \times \frac{{63.55}}{2}\)
D. \(\frac{{1.00}}{{107.87}} \times 63.55\)
Which components are used to make the standard hydrogen electrode?
A. \({{\text{H}}_{\text{2}}}{\text{(g), }}{{\text{H}}^ + }{\text{(aq), Pt(s)}}\)
B. \({{\text{H}}_{\text{2}}}{\text{(g), }}{{\text{H}}^ + }{\text{(aq), Ni(s)}}\)
C. \({{\text{H}}_{\text{2}}}{\text{(g), H}}{{\text{O}}^ - }{\text{(aq), Pt(s)}}\)
D. \({{\text{H}}_{\text{2}}}{\text{(g), H}}{{\text{O}}^ - }{\text{(aq), Ni(s)}}\)
A voltaic cell is made by connecting two half-cells represented by the half-equations below.
\[\begin{array}{*{20}{l}} {{\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Mn(s)}}}&{{E^\Theta } = - 1.19{\text{ V}}} \\ {{\text{P}}{{\text{b}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {\text{Pb(s)}}}&{{E^\Theta } = - 0.13{\text{ V}}} \end{array}\]
Which statement is correct about this voltaic cell?
A. Mn is oxidized and the voltage of the cell is 1.06 V.
B. Pb is oxidized and the voltage of the cell is 1.06 V.
C. Mn is oxidized and the voltage of the cell is 1.32 V.
D. Pb is oxidized and the voltage of the cell is 1.32 V.
The overall equation of a voltaic cell is:
\[{\text{Ni(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \rightleftharpoons {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}\;\;\;\;\;{E^\Theta } = {\text{1.06 V}}\]
The standard electrode potential for \({\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Ni(s)}}\), is \( - 0.26{\text{ V}}\). What is the standard electrode potential for the silver half-cell, \({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{Ag(s)}}\), in V?
A. \( - 1.32\)
B. \( - 0.80\)
C. \( + 0.80\)
D. \( + 1.32\)
The same quantity of electricity was passed through separate molten samples of sodium bromide, NaBr, and magnesium chloride, \({\text{MgC}}{{\text{l}}_{\text{2}}}\). Which statement is true about the amounts, in mol, that are formed?
A. The amount of Mg formed is equal to the amount of Na formed.
B. The amount of Mg formed is equal to the amount of \({\text{C}}{{\text{l}}_{\text{2}}}\) formed.
C. The amount of Mg formed is twice the amount of \({\text{C}}{{\text{l}}_{\text{2}}}\) formed.
D. The amount of Mg formed is twice the
What does not affect the mass of products formed in electrolysis of an aqueous solution?
A. Current
B. Duration of electrolysis
C. Initial mass of cathode
D. Charge on the ions
Consider the following two standard electrode potentials at 298 K.
\({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Sn(s)}}\) \({E^\Theta } = - 0.14{\text{ V}}\)
\({\text{F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 0.77{\text{ V}}\)
What is the equation and cell potential for the spontaneous reaction that occurs?
A. \({\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}} \to {\text{2F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{Sn(s)}}\) \({E^\Theta } = - 0.91{\text{ V}}\)
B. \({\text{2F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{Sn(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 0.91{\text{ V}}\)
C. \({\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}} \to {\text{2F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{Sn(s)}}\) \({E^\Theta } = + 0.91{\text{ V}}\)
D. \({\text{2F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{Sn(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) \({E^\Theta } = + 1.68{\text{ V}}\)
For the electrolysis of aqueous copper(II) sulfate, which of the following statements is correct?
A. Cu and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 1:1
B. \({{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 1:1
C. Cu and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 2:1
D. \({{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\) are produced in a mol ratio of 2:1
What are the products of electrolysis when concentrated calcium bromide solution is electrolysed using graphite electrodes?