
SL Paper 2
Chlorine occurs in Group 7, the halogens.
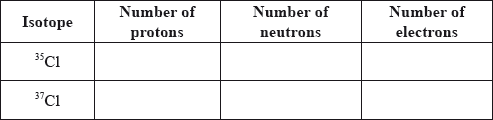
Two stable isotopes of chlorine are \(^{{\text{35}}}{\text{Cl}}\) and \(^{{\text{37}}}{\text{Cl}}\) with mass numbers 35 and 37 respectively.
Chlorine has an electronegativity value of 3.2 on the Pauling scale.
Chloroethene, H2C=CHCl, the monomer used in the polymerization reaction in the manufacture of the polymer poly(chloroethene), PVC, can be synthesized in the following two-stage reaction pathway.
\[\begin{array}{*{20}{l}} {{\text{Stage 1:}}}&{{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}}} \\ {{\text{Stage 2:}}}&{{\text{ClC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Cl(g)}} + {\text{HC=CHCl(g)}} + {\text{HCl(g)}}} \end{array}\]
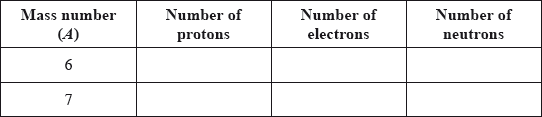
Define the term isotopes of an element.
Calculate the number of protons, neutrons and electrons in the isotopes 35Cl and 37Cl.
Using the mass numbers of the two isotopes and the relative atomic mass of chlorine from Table 5 of the Data Booklet, determine the percentage abundance of each isotope.
Percentage abundance 35Cl:
Percentage abundance 37Cl:
Define the term electronegativity.
Using Table 7 of the Data Booklet, explain the trends in electronegativity values of the Group 7 elements from F to I.
State the balanced chemical equation for the reaction of potassium bromide, KBr(aq), with chlorine, Cl2(aq).
Describe the colour change likely to be observed in this reaction.
Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for stage 1 using average bond enthalpy data from Table 10 of the Data Booklet.
State whether the reaction given in stage 1 is exothermic or endothermic.
Draw the structure of poly(chloroethene) showing two repeating units.
Suggest why monomers are often gases or volatile liquids whereas polymers are solids.
Table 8 of the Data Booklet shows the atomic and ionic radii of the elements.
Describe and explain the trend in atomic radius across period 3.
A student formulates the following hypothesis: “If phosphorus were to form a positive ion, \({{\text{P}}^{3 + }}\), its ionic radius would probably be between \({\text{110}} \times {\text{1}}{{\text{0}}^{ - 12}}{\text{ m}}\) and \({\text{212}} \times {\text{1}}{{\text{0}}^{ - 12}}{\text{ m}}\).” Evaluate this hypothesis.
Sodium oxide, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\), is a white solid with a high melting point.
Explain why solid sodium oxide is a non-conductor of electricity.
Molten sodium oxide is a good conductor of electricity. State the half-equation for the reaction occurring at the positive electrode during the electrolysis of molten sodium oxide.
State the acid-base nature of sodium oxide.
State the equation for the reaction of sodium oxide with water.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
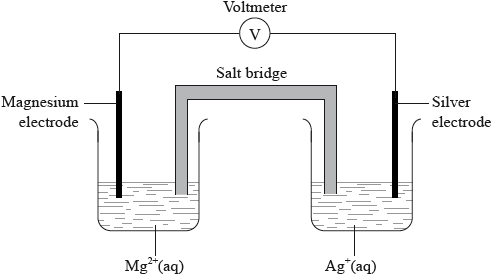
A voltaic cell is made from a half-cell containing a magnesium electrode in a solution of magnesium nitrate and a half-cell containing a silver electrode in a solution of silver(I) nitrate.
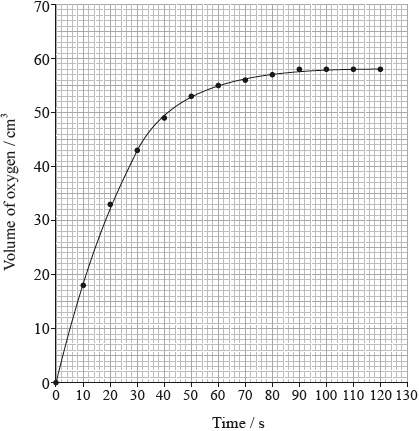
Hydrogen peroxide decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
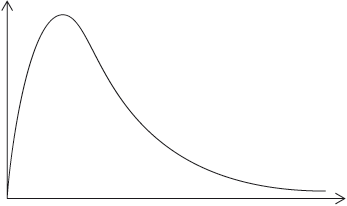
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
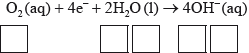
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
(i) Given that magnesium is more reactive than silver, deduce the half-equations for the reactions occurring at each electrode, including state symbols.
Negative electrode (anode):
Positive electrode (cathode):
(ii) Outline one function of the salt bridge.
(i) State the property that determines the order in which elements are arranged in the periodic table.
(ii) State the relationship between the electron arrangement of an element and its group and period in the periodic table.
(i) The experiment is repeated with the same amount of a more effective catalyst, \({\text{Mn}}{{\text{O}}_{\text{2}}}\), under the same conditions and using the same concentration and volume of hydrogen peroxide. On the graph above, sketch the curve you would expect.
(ii) Outline how the initial rate of reaction can be found from the graph.
(iii) Outline a different experimental procedure that can be used to monitor the decomposition rate of hydrogen peroxide.
(iv) A Maxwell–Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
Oxidation and reduction can be defined in terms of electron transfer or oxidation numbers.
Alcohols with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) occur as four structural isomers. Three of the isomers can be oxidized with acidified potassium dichromate solution to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). The half-equation for the dichromate ion is:
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \rightleftharpoons {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\]
Electrolysis has made it possible to obtain reactive metals from their ores.
A reactivity series can be experimentally determined by adding the metals W, X, Y and Z to solutions of these metal ions. The following reactions were observed:
\({{\text{W}}^{2 + }}{\text{(aq)}} + {\text{X(s)}} \to {\text{W(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{W(s)}}\)
\({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{W(s)}} \to {\text{Z(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{X(s)}}\)
Define oxidation in terms of electron transfer.
(i) Deduce the oxidation number of chromium in \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }\).
(ii) Deduce the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(iii) Deduce the overall equation for the redox reaction.
(iv) Two of the isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) can be oxidized further to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). Deduce the structural formulas of these two isomers.
(v) One isomer cannot be oxidized by acidified potassium dichromate solution.
Deduce its structural formula, state its name and identify it as a primary, secondary or tertiary alcohol.
Name:
Alcohol:
(vi) All isomers of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) undergo complete combustion. State an equation for the complete combustion of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(i) Draw a labelled electrolytic cell for the electrolysis of molten potassium bromide, KBr. Include the direction of electron flow, the positive electrode (anode) and the negative electrode (cathode), the location of oxidation and reduction, and the electrolyte.
(ii) Deduce a half-equation for the reaction that occurs at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(iii) Describe how current is conducted in a molten electrolyte.
(i) Deduce the order of reactivity of these four metals, from the least to the most reactive.
(ii) A voltaic cell is made by connecting a half-cell of X in \({\text{XC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\) to a half-cell of Z in \({\text{ZC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\). Deduce the overall equation for the reaction taking place when the cell is operating.
Draw the Lewis (electron dot) structure of chloromethane.
Predict the shape of the chloromethane molecule and the H–C–H bond angle.
Shape:
Bond angle:
Explain why chloromethane is a polar molecule.
Methanol has a lower molar mass than chloromethane. Explain why the boiling point of methanol is higher than that of chloromethane.
State the equation for the reaction between potassium and chlorine.
Outline the nature of the metallic bonding present in potassium.
Describe the covalent bond present in the chlorine molecule and how it is formed.
Describe the ionic bonding present in potassium chloride and how the ions are formed.
Potassium also reacts with water to form hydrogen gas. Determine the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of hydrogen gas that could theoretically be produced at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\) when 0.0587 g of potassium reacts with excess water.
Identify the acid-base character of the oxides of each of the elements from sodium to chlorine in period 3.
State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with water.
Across period 3, elements increase in atomic number, decrease in atomic radius and increase in electronegativity.
Define the term electronegativity.
Explain why the atomic radius of elements decreases across the period.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Suggest the pH of the solutions formed in part (c) (i).
Describe three tests that can be carried out in the laboratory, and the expected results, to distinguish between \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) and \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\).
Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
Describe the bonding and structure of sodium chloride.
State the formula of the compounds formed between the elements below.
Sodium and sulfur:
Magnesium and phosphorus:
Covalent bonds form when phosphorus reacts with chlorine to form \({\text{PC}}{{\text{l}}_{\text{3}}}\). Deduce the Lewis (electron dot) structure, the shape and bond angle in \({\text{PC}}{{\text{l}}_{\text{3}}}\) and explain why the molecule is polar.
Lewis (electron dot) structure:
Name of shape:
Bond angle:
Explanation of polarity of molecule:
Periodic trends enable chemists to predict the behaviour of related compounds.
Chlorine gas, \({\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\), is bubbled through separate solutions of aqueous bromine, \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\), and potassium bromide, \({\text{KBr(aq)}}\).
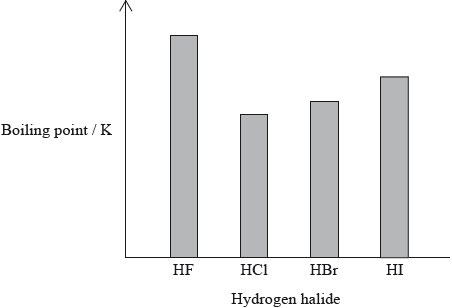
The hydrogen halides do not show perfect periodicity. A bar chart of boiling points shows that the boiling point of hydrogen fluoride, HF, is much higher than periodic trends would indicate.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) are two oxides of period 3 elements.
(i) State the equation for the reaction of sodium metal with water.
(ii) Describe two changes that could be observed during the reaction.
(iii) Predict the relative reaction rates of lithium, sodium and potassium with water.
(i) Predict any changes that may be observed in each case.
\({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\):
\({\text{KBr(aq)}}\):
(ii) State the half-equations for the reactions that occur.
(i) Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
(ii) Explain the trend in the boiling points of HCl, HBr and HI.
Explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) does not conduct electricity in the solid state but it does when molten. Pure \({\text{S}}{{\text{O}}_{\text{3}}}\) does not conduct electricity in either the solid or liquid states.
Explain these facts.
State the acid-base natures of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\).
State equations for the reactions of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) with water.
The relative atomic mass of naturally occurring copper is 63.55. Calculate the abundances of \(^{{\text{63}}}{\text{Cu}}\) and \(^{{\text{65}}}{\text{Cu}}\) in naturally occurring copper.
The isotopes of some elements are radioactive. State a radioisotope used in medicine.
State a balanced equation for the reaction of sodium with water. Include state symbols.
With reference to electronic arrangements, suggest why the reaction between rubidium and water is more vigorous than that between sodium and water.
Describe and explain what you will see if chlorine gas is bubbled through a solution of
(i) potassium iodide.
(ii) potassium fluoride.
Calcium nitrate contains both covalent and ionic bonds.
Nitrogen also forms oxides, which are atmospheric pollutants.
State the formula of both ions present and the nature of the force between these ions.
Ions:
Nature of force:
State which atoms are covalently bonded.
Outline the source of these oxides.
State one product formed from their reaction with water.
State one environmental problem caused by these atmospheric pollutants.
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
(i) Define the terms atomic number, mass number and isotopes of an element.
Atomic number:
Mass number:
Isotopes of an element:
(ii) Distinguish between the terms group and period.
(iii) Deduce the electron arrangements of the lithium ion, \({\text{L}}{{\text{i}}^ + }\), and the boron atom, B.
\({\text{L}}{{\text{i}}^ + }\):
B:
(iv) Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
v) Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for each isotope.
(i) Distinguish between a continuous spectrum and a line spectrum.
(ii) Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the ultraviolet and visible regions of the spectrum. Label your diagram to show three transitions for each series.
(i) Explain why metals are good conductors of electricity and why they are malleable.
(ii) Iron is described as a transition metal. Identify the two most common ions of iron.
iii) Deduce the chemical formulas of lithium oxide and iron(II) oxide.
Lithium oxide:
Iron(II) oxide:
Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and water.
(i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol phosphine. Calculate the temperature rise using section 1 of the data booklet and the data below.
Standard enthalpy of combustion of phosphine,
Specific heat capacity of air = 1.00Jg−1K−1 = 1.00 kJkg−1K−1
(ii) The oxide formed in the reaction with air contains 43.6 % phosphorus by mass. Determine the empirical formula of the oxide, showing your method.
(iii) The molar mass of the oxide is approximately 285gmol−1. Determine the molecular formula of the oxide.
(i) State the equation for the reaction of this oxide of phosphorus with water.
(ii) Predict how dissolving an oxide of phosphorus would affect the pH and electrical conductivity of water.
pH:
Electrical conductivity:
(iii) Suggest why oxides of phosphorus are not major contributors to acid deposition.
(iv) The levels of sulfur dioxide, a major contributor to acid deposition, can be minimized by either pre-combustion and post-combustion methods. Outline one technique of each method.
Pre-combustion:
Post-combustion:
The element antimony, Sb, is usually found in nature as its sulfide ore, stibnite, \({\text{S}}{{\text{b}}_{\text{2}}}{{\text{S}}_{\text{3}}}\). This ore was used two thousand years ago by ancient Egyptian women as a cosmetic to darken their eyes and eyelashes.
One method of extracting antimony from its sulfide ore is to roast the stibnite in air. This forms antimony oxide and sulfur dioxide. The antimony oxide is then reduced by carbon to form the free element.
Deduce the oxidation number of antimony in stibnite.
Deduce one other common oxidation number exhibited by antimony in some of its compounds.
Deduce the chemical equations for these two reactions.
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
Phosphorus(V) oxide, \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{ }}({M_{\text{r}}} = 283.88)\), reacts vigorously with water \(({M_{\text{r}}} = 18.02)\), according to the equation below.
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{(s)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4}}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\]
Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
Describe the bonding in magnesium.
State an equation for the reaction of magnesium oxide with water.
A student added 5.00 g of \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) to 1.50 g of water. Determine the limiting reactant, showing your working.
Calculate the mass of phosphoric(V) acid, \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\), formed in the reaction.
State a balanced equation for the reaction of aqueous \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\) with excess aqueous sodium hydroxide, including state symbols.
State the formula of the conjugate base of \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\).
(i) Deduce the Lewis structure of \({\text{PH}}_4^ + \).
(ii) Predict, giving a reason, the bond angle around the phosphorus atom in \({\text{PH}}_4^ + \).
(iii) Predict whether or not the P–H bond is polar, giving a reason for your choice.
The periodic table shows the relationship between electron arrangement and the properties of elements and is a valuable tool for making predictions in chemistry.
The word redox comes from a combination of the terms reduction and oxidation. Redox reactions affect our daily lives.
The overall reaction that takes place in a voltaic cell is shown below.
\[{\text{Pb(s)}} + {\text{Pb}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}} \to {\text{2PbS}}{{\text{O}}_{\text{4}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Identify the property used to arrange the elements in the periodic table.
(ii) Outline two reasons why electronegativity increases across period 3 in the periodic table and one reason why noble gases are not assigned electronegativity values.
(i) Define the term first ionization energy of an atom.
(ii) Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
(iii) Explain why sodium conducts electricity but phosphorus does not.
(i) Determine the oxidation number of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\).
(ii) Deduce the oxidation and reduction half-equations taking place at the negative lead electrode (anode) and the positive lead(IV) oxide electrode (cathode). Deduce the oxidizing and reducing agents and state the direction of the electron flow between the electrodes.
(iii) In order to determine the position of three metals in a reactivity series, the metals were placed in different solutions of metal ions. The table below summarizes whether or not a reaction occurred.
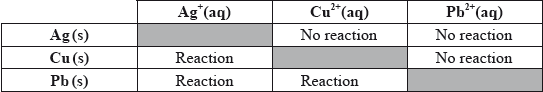
State the equations for the three reactions that take place. Use this information to place the metals Ag, Cu and Pb in a reactivity series, with the strongest reducing agent first, and explain your reasoning.
Consider the bonding and structure of the period 3 elements.
Explain the increase in the melting point from sodium to aluminium.
Explain why sulfur, \({{\text{S}}_{\text{8}}}\), has a higher melting point than phosphorus, \({{\text{P}}_{\text{4}}}\).
Explain why silicon has the highest melting point and argon has the lowest melting point.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
State the electron configuration of the Ca2+ ion.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Explain why:
Define the term first ionization energy.
Explain why the first ionization energy of magnesium is higher than that of sodium.
calcium has a higher melting point than potassium.
sodium oxide has a higher melting point than sulfur trioxide.
Define the terms acid and base according to the Brønsted-Lowry theory and state one example of a weak acid and one example of a strong base.
Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.
Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) is greater in the more acidic product.
Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
Define the term isotopes.
A sample of silicon contains three isotopes.

Calculate the relative atomic mass of silicon using this data.
Describe the structure and bonding in silicon dioxide and carbon dioxide.
Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle in \({\text{N}}{{\text{H}}_{\text{3}}}\).
The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in the boiling points.

Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
The graph of the first ionization energy plotted against atomic number for the first twenty elements shows periodicity.
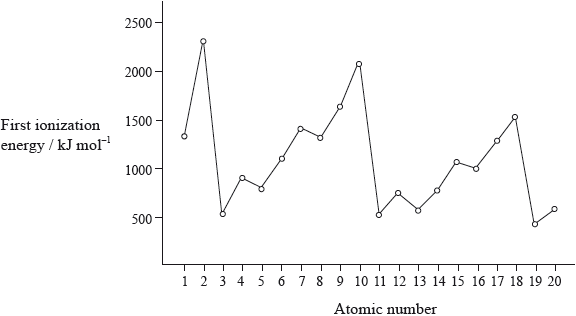
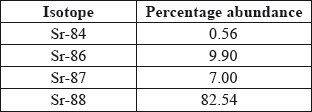
Strontium exists as four naturally-occurring isotopes. Calculate the relative atomic mass of strontium to two decimal places from the following data.
Define the term first ionization energy and state what is meant by the term periodicity.
State the electron arrangement of argon and explain why the noble gases, helium, neon and argon show the highest first ionization energies for their respective periods.
A graph of atomic radius plotted against atomic number shows that the atomic radius decreases across a period. Explain why chlorine has a smaller atomic radius than sodium.
Explain why a sulfide ion, \({{\text{S}}^{2 - }}\), is larger than a chloride ion, \({\text{C}}{{\text{l}}^ - }\).
Explain why the melting points of the Group 1 metals \({\text{(Li}} \to {\text{Cs)}}\) decrease down the group whereas the melting points of the Group 7 elements \({\text{(F}} \to {\text{I)}}\) increase down the group.
State the equation for the reaction between sodium and water.
State and explain one difference between the reactions of sodium and potassium with water.
Some of the most important processes in chemistry involve acid-base reactions.
Describe the acid-base character of the oxides of each of the period 3 elements, Na to Cl.
State one example of an acidic gas, produced by an industrial process or the internal combustion engine, which can cause large-scale pollution to lakes and forests.
Suggest one method, other than measuring pH, which could be used to distinguish between solutions of a strong acid and a weak acid of the same molar concentration. State the expected results.
Ethene, C2H4, and hydrazine, N2H4, are hydrides of adjacent elements in the periodic table.
The polarity of a molecule can be explained in terms of electronegativity.
The reaction between N2H4(aq) and HCl (aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw Lewis (electron dot) structures for \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) and \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) showing all valence electrons.
(ii) State and explain the H–C–H bond angle in ethene and the H–N–H bond angle in hydrazine.
(i) Define the term electronegativity.
(ii) Compare the relative polarities of the C–H bond in ethene and the N–H bond in hydrazine.
(iii) Hydrazine is a polar molecule and ethene is non-polar. Explain why ethene is non-polar.
The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
Hydrazine is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(g)}}\]
Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
State the name of the product and identify the type of reaction which occurs between ethene and hydrogen chloride.
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_6^{2 + }\).
Carbon and silicon belong to the same group of the periodic table.
Both silicon and carbon form oxides.
State the period numbers of both carbon and silicon.
Describe and compare three features of the structure and bonding in the three allotropes of carbon: diamond, graphite and \({{\text{C}}_{{\text{60}}}}\) fullerene.
Draw the Lewis structure of \({\text{C}}{{\text{O}}_{\text{2}}}\) and predict its shape and bond angle.
Describe the structure and bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\).
Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
Describe the bonding within the carbon monoxide molecule.
Silicon has three stable isotopes, \(^{{\text{28}}}{\text{Si}}\), \(^{{\text{29}}}{\text{Si}}\) and \(^{{\text{30}}}{\text{Si}}\). The heaviest isotope, \(^{{\text{30}}}{\text{Si}}\), has a percentage abundance of 3.1%. Calculate the percentage abundance of the lightest isotope to one decimal place.
Phosphorus tribromide (\({\text{PB}}{{\text{r}}_{\text{3}}}\)) is used to manufacture alprazolam, a drug used to treat anxiety disorders. Methanal (HCHO) is used as a disinfectant.
Consider the following reaction sequence:
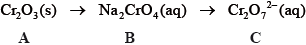
Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the electron arrangements of the reactants and product, and explain whether sulfur is oxidized or reduced.
Describe the acid-base character of the oxides of the period 3 elements, Na to Cl. For the compounds sodium oxide and phosphorus(V) oxide, state the balanced chemical equations for the reaction of each oxide with water.
For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO:
• deduce the Lewis structure.
• predict the shape and bond angle.
Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
State the name of A.
Describe the redox behaviour of chromium with reference to oxidation numbers in the conversion of B to C.
Define the term oxidizing agent and identify the oxidizing agent in the following
reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
Airbags are an important safety feature in vehicles. Sodium azide, potassium nitrate and silicon dioxide have been used in one design of airbag.

Sodium azide, a toxic compound, undergoes the following decomposition reaction under certain conditions.
\[{\text{2Na}}{{\text{N}}_{\text{3}}}{\text{(s)}} \to {\text{2Na(s)}} + {\text{3}}{{\text{N}}_{\text{2}}}{\text{(g)}}\]
Two students looked at data in a simulated computer-based experiment to determine the volume of nitrogen generated in an airbag.
Using the simulation programme, the students entered the following data into the computer.
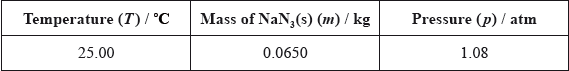
The chemistry of the airbag was found to involve three reactions. The first reaction involves the decomposition of sodium azide to form sodium and nitrogen. In the second reaction, potassium nitrate reacts with sodium.
\[{\text{2KN}}{{\text{O}}_3}{\text{(s)}} + {\text{10Na(s)}} \to {{\text{K}}_2}{\text{O(s)}} + {\text{5N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{N}}_2}{\text{(g)}}\]
An airbag inflates very quickly.
Sodium azide involves ionic bonding, and metallic bonding is present in sodium. Describe ionic and metallic bonding.
State the number of significant figures for the temperature, mass and pressure data.
T:
m:
p:
Calculate the amount, in mol, of sodium azide present.
Determine the volume of nitrogen gas, in \({\text{d}}{{\text{m}}^{\text{3}}}\), produced under these conditions based on this reaction.
Suggest why it is necessary for sodium to be removed by this reaction.
The metal oxides from the second reaction then react with silicon dioxide to form a silicate in the third reaction.
\[{{\text{K}}_2}{\text{O(s)}} + {\text{N}}{{\text{a}}_2}{\text{O(s)}} + {\text{Si}}{{\text{O}}_2}{\text{(s)}} \to {\text{N}}{{\text{a}}_2}{{\text{K}}_2}{\text{Si}}{{\text{O}}_4}{\text{(s)}}\]
Draw the structure of silicon dioxide and state the type of bonding present.
Structure:
Bonding:
It takes just 0.0400 seconds to produce nitrogen gas in the simulation. Calculate the average rate of formation of nitrogen in (b) (iii) and state its units.
The students also discovered that a small increase in temperature (e.g. 10 °C) causes a large increase (e.g. doubling) in the rate of this reaction. State one reason for this.
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, \({}_Z^AX\), for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the \({}_{22}^{48}{\text{Ti}}\) atom.
State the full electron configuration of the \({}_{22}^{48}{\text{Ti}}\)2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.