
SL Paper 2
Explain why:
Define the term first ionization energy.
Explain why the first ionization energy of magnesium is higher than that of sodium.
calcium has a higher melting point than potassium.
sodium oxide has a higher melting point than sulfur trioxide.
Define the terms acid and base according to the Brønsted-Lowry theory and state one example of a weak acid and one example of a strong base.
Describe two different methods, one chemical and one physical, other than measuring the pH, that could be used to distinguish between ethanoic acid and hydrochloric acid solutions of the same concentration.
Black coffee has a pH of 5 and toothpaste has a pH of 8. Identify which is more acidic and deduce how many times the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) is greater in the more acidic product.
Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce the equation for each reaction and identify each oxide as acidic, basic or neutral.
Markscheme
the amount of energy required to remove one (mole of) electron(s);
from (one mole of) an atom(s) in the gaseous state;
greater positive charge on nucleus / greater number of protons / greater core charge;
greater attraction by Mg nucleus for electrons (in the same shell) / smaller atomic radius;
calcium ionic charge is twice/greater than the potassium ionic charge / calcium has more delocalized electrons than potassium;
greater attraction of delocalized electrons and \({\text{C}}{{\text{a}}^{2 + }}\) / less attraction between the delocalized electrons and \({{\text{K}}^ + }\);
Do not accept calcium ion has a 2+ without comparison to \({{\text{K}}^ + }\).
Na2O ionic/(stronger electrostatic) attractions between \({\text{N}}{{\text{a}}^ + }\) and \({{\text{O}}^{2 - }}\);
\({\text{S}}{{\text{O}}_{\text{3}}}\) has (weak) intermolecular/van der Waals’/London/dispersion/dipoledipole attractions;
intermolecular/van der Waals’/London/dispersion/dipole-dipole forces are weaker/more easily broken than (strong) ionic bonds / ionic bonds are stronger/harder to break than intermolecular bond/van der Waals’/London/dispersion/dipole-dipole forces;
acid is a proton/\({{\text{H}}^ + }\) donor and base is a proton/\({{\text{H}}^ + }\) acceptor;
\({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\)/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and NaOH/KOH/\({\text{Ba(OH}}{{\text{)}}_{\text{2}}}\);
Accept any suitable examples.
Chemical
reaction with reactive metal/Mg/Zn/carbonate/hydrogen carbonate;
hydrochloric acid would react faster/more vigorously / ethanoic acid would react slower/less vigorously;
OR
react with alkali;
temperature change will be more for hydrochloric acid / temperature change will be less for ethanoic acid;
Physical
conductivity;
hydrochloric acid will conduct more/higher / ethanoic acid will conduct less/lower;
Accept other suitable examples.
black coffee;
\({\text{1}}{{\text{0}}^{\text{3}}}\)/1000 times;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({\text{S}}{{\text{O}}_3}{\text{(l)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\): basic and \({\text{S}}{{\text{O}}_{\text{3}}}\): acidic;
Examiners report
The definition of first ionisation energy given by most candidates in (a) (i) was incomplete. The word gaseous was missing from most definitions given.
Candidates also struggled to explain the differences in first ionization energies of magnesium and sodium. Candidates did not need knowledge of subshells as was suggested in one comment in the G2 forms. Candidates needed to make reference to nuclear charge and size of atomic radius and their effect on the attraction to the electrons.
Part (b) (i) clearly indicated that candidates were not familiar with metallic bonding.
In part (ii) the candidates incorrectly discussed the bonding in the sulfur trioxide molecule rather than the intermolecular forces. Many candidates incorrectly wrote words to the effect that ionic bonding was stronger than covalent bonding to explain the differences in melting point of the two compounds.
Parts (c) (i) and (iii) were well managed with candidates correctly defining acids and bases according to the Brønsted-Lowry theory and had a good understanding of the relationship between pH and concentration of \({{\text{H}}^ + }\) ions.
Part (c) (ii) was reasonably well answered but candidates did not always provided one chemical and one physical method to distinguish between the two acids.
Even though candidates were able to identify sodium oxide and sulfur trioxide as basic and acidic respectively they struggled to write correct equations for the oxides with water in part (d).
Chloroethene, C2H3Cl, is an important organic compound used to manufacture the polymer poly(chloroethene).
Draw the Lewis structure for chloroethene and predict the H–C–Cl bond angle.
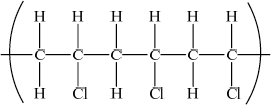
Draw a section of poly(chloroethene) containing six carbon atoms.
Outline why the polymerization of alkenes is of economic importance and why the disposal of plastics is a problem.
Chloroethene can be converted to ethanol in two steps. For each step deduce an overall equation for the reaction taking place.
Step 1:
Step 2:
State the reagents and conditions necessary to prepare ethanoic acid from ethanol in the laboratory.
State an equation, including state symbols, for the reaction of ethanoic acid with water. Identify a Brønsted-Lowry acid in the equation and its conjugate base.
Markscheme
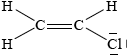 ;
;
Accept lines, dots or crosses for electron pairs.
Lone pairs required on chlorine.
(approximately) 120°;
Accept any bond angle in the range 113–120°.
 ;
;
Brackets not required for mark.
Continuation bonds from each carbon are required.
Cl atoms can be above or below carbon spine or alternating above and below.
plastics are cheap/versatile/a large industry / plastics have many uses / OWTTE;
plastics are not biodegradeable / plastics take up large amounts of space in landfill / pollution caused by burning of plastics / OWTTE;
Do not accept plastics cause litter.
Allow plastics don’t decompose quickly / OWTTE.
(i) Step 1:
\({\text{C}}{{\text{H}}_2}{\text{CHCl}} + {{\text{H}}_2} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{Cl}}\);
Step 2:
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{Cl}} + {\text{O}}{{\text{H}}^ - } \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} + {\text{C}}{{\text{l}}^ - }\);
Allow NaOH or NaCl etc. instead of OH– and Cl–.
Allow abbreviated formulas C2H3Cl, C2H5Cl, C2H5OH.
\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\)/\({{\text{H}}^ + }\)/acidified and \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{_{\text{7}}}^{2 - }\)/(potassium/sodium) dichromate;
Accept suitable oxidizing agents (e.g. KMnO4 etc.) but only with acid.
Ignore missing or incorrect oxidation states in reagents.
(heat under) reflux;
Second mark can be scored even if reagent is incorrect.
\({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}\)
OR
\({\text{C}}{{\text{H}}_3}{\text{COOH(l)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}}\)
OR
\({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\)
correct equation;
state symbols and \( \rightleftharpoons \);
BL acid is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) and cb is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}{{\text{O}}^ - }\) / BL acid is \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) and cb is \({{\text{H}}_{\text{2}}}{\text{O}}\);
Examiners report
The main G2 comments on this question related to the inclusion of organic chemistry in Section A. It should be noted that ANY Topic can be asked in Section A of P2, and there is no set-formula in relation to question setting. Organic chemistry is an integral part of the IB SL Chemistry programme, and is covered in Topic 10 of the guide (12 hours in total). Hence, candidates should be adequately prepared for questions on this topic, even in Section A. In 3(a), the Lewis structure of chlorethene was generally drawn correctly, though the weaker candidates often omitted the lone pairs on the chlorine. The bond angle was usually predicted, although right angles and 109.5° were often given. Even some of the better candidates explained their choice of bond angle, based on the fact that the double bond occupies more space causing the HCCl bond angle to drop less than 120°.
Many candidates gave double bonds and some forgot to include continuation bonds.
The Aim 8 question in part (iii) was very well answered this session. Almost all candidates scored the disposal problem of plastics mark and many achieved the economics importance mark also.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. (i) was poorly done. Candidates who managed a correct reaction for the first step often used water instead of hydroxide ion for the second step.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. In (ii), candidates who mentioned dichromate(VI) or permanganate(VIII) often omitted the acid. In addition, reflux was often missing.
In general (b) was very poorly answered, again showing a clear weakness in organic chemistry, which is an area of major concern. In (iii), very few candidates scored all three marks here, even though the question itself was easy. The equation was often correct, but the equilibrium arrow was rarely given. Some candidates did not know the formula for ethanoic acid which was surprising.
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.
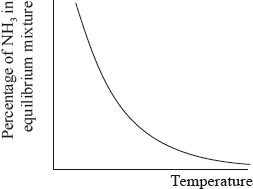
Fertilizers may cause health problems for babies because nitrates can change into nitrites in water used for drinking.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Explain the effect of increasing the temperature on the rate of reaction.
(i) Define oxidation in terms of oxidation numbers.
(ii) Deduce the oxidation states of nitrogen in the nitrate, \({\text{NO}}_{\text{3}}^ - \), and nitrite, \({\text{NO}}_{\text{2}}^ - \), ions.
The nitrite ion is present in nitrous acid, HNO2, which is a weak acid. The nitrate ion is present in nitric acid, HNO3, which is a strong acid. Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
A small piece of magnesium ribbon is added to solutions of nitric and nitrous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since nitrous acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
The graph below shows how the conductivity of the two acids changes with concentration.
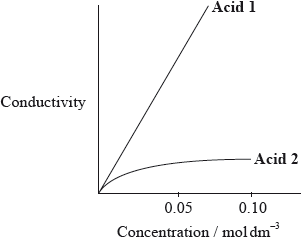
Identify Acid 1 and explain your choice.
Nitric acid reacts with silver in a redox reaction.
__ \({\text{Ag(s)}} + \) __ \({\text{NO}}_3^ - {\text{(aq)}} + \) ___ \( \to \) ___\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + \) __ \({\text{NO(g)}} + \) ____
Using oxidation numbers, deduce the complete balanced equation for the reaction showing all the reactants and products.
Markscheme
(i) exothermic;
Accept either of the following for the second mark.
increasing temperature favours endothermic/reverse reaction;
as yield decreases with increasing temperature;
(ii) yield increases / equilibrium moves to the right / more ammonia;
increase in pressure favours the reaction which has fewer moles of gaseous products;
(iii) (rate increases because) increase in frequency (of collisions);
increase in energy (of collisions);
more colliding molecules with \(E \geqslant {E_{\text{a}}}\);
(i) increase in the oxidation number;
(ii) (NO3) + 5 and (NO2–) + 3;
Accept V and III.
Do not penalize missing charges on numbers.
strong acid completely dissociated/ionized and weak acid partially dissociated/ionized;
\({\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_3^ - {\text{(aq)}}\);
\({\text{HN}}{{\text{O}}_2}{\text{(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{NO}}_2^ - {\text{(aq)}}\);
Allow only arrows as shown.
State symbols not needed.
Accept H2O and H3O+.
With HNO3:
faster rate of bubble/gas/hydrogen production;
faster rate of magnesium dissolving;
higher temperature change;
Accept opposite argument for HNO2.
Award [1] if 2 observations given but acid is not identified.
Reference to specific observations needed.
(i) (nitric acid) \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
(ii) not valid as nitrous acid reacts with same volume/ \({\text{7.5 c}}{{\text{m}}^{\text{3}}}\);
HNO3;
(higher conductivity for solutions with same concentration as) there are more ions in solution;
change in oxidation numbers: Ag from 0 to +1 and N from +5 to +2;
Do not penalise missing charges on numbers.
balanced equation: \({\text{3Ag}} + {\text{NO}}_3^ - + {\text{4}}{{\text{H}}^ + } \to {\text{3A}}{{\text{g}}^ + } + {\text{NO}} + {\text{2}}{{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and product;
Award [3] for correct balanced equation.
Ignore state symbols
Examiners report
This was the most popular question and it was well answered by the majority of candidates. The reaction was correctly described as exothermic and the reason for this explained correctly in most cases. Most candidates knew that yield would increase with increased pressure, but failed to score a second mark because they did not mention ‘gaseous’ although they did know the answer. The effect of increased temperature on rate was generally well described although some did get confused with yield and how it would affect equilibrium.
Most candidates correctly defined oxidation in 6(b)(i) but ‘hedged their bets’ by stating loss of electrons as well as an increase in oxidation number. In 6(b)(ii) the oxidation states were generally deduced correctly but sometimes written as ionic charges (5+ for instance, instead of +5).
In 6(c) most correctly defined strong and weak acids, and many also wrote correct equations. A few, though, had no idea. In (c), arrows proved to be a minefield for several candidates, especially the equilibrium sign. HA was commonly given, as were CH3COOH and HCl, instead of nitric and nitrous acid.
6(d) presented problems with many candidates unable to describe observations and instead stating there would be ‘more hydrogen produced’ or just that ‘the reaction would be faster’. However, better candidates were able to answer this part correctly and scored full marks.
In 6(e)(i) the calculation was answered well, but 6(e)(ii), that asked for a comment on the hypothesis, was not and few candidates stated that the same volume of acid was needed.
In 6(f), the majority correctly identified the strong acid but often failed to explain its better conductivity in terms of the ions.
Many could give a correct balanced equation and scored the 3 marks, and others scored 1 mark for giving the correct reactants and products. However, not many candidates used oxidation numbers to deduce the balanced equation.
Arsenic and nitrogen play a significant role in environmental chemistry. Arsenous acid, H3AsO3, can be found in oxygen-poor (anaerobic) water, and nitrogen-containing fertilizers can contaminate water.
Nitric acid, HNO3, is strong and nitrous acid, HNO2, is weak.
(i) Define oxidation and reduction in terms of electron loss or gain.
Oxidation:
Reduction:
(ii) Deduce the oxidation numbers of arsenic and nitrogen in each of the following species.
\({\text{A}}{{\text{s}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
\({\text{NO}}_3^ - \):
\({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\):
\({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
(iii) Distinguish between the terms oxidizing agent and reducing agent.
(iv) In the removal of arsenic from contaminated groundwater, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\) is often first oxidized to arsenic acid, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
The following unbalanced redox reaction shows another method of forming \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
\[{\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{NO}}_3^ - {\text{(aq)}} \to {{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\]
Deduce the balanced redox equation in acid, and then identify both the oxidizing and reducing agents.
Define an acid according to the Brønsted–Lowry and Lewis theories.
Brønsted–Lowry theory:
Lewis theory:
The Lewis (electron dot) structure of nitrous acid is given below.

Identify which nitrogen-oxygen bond is the shorter.
Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and explain your answer.
Distinguish between a strong acid and a weak acid in terms of their dissociation in aqueous solution.
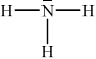
Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the name of the shape of the molecule and explain why NH3 is a polar molecule.
When lime was added to a sample of soil, the pH changed from 5 to 7. Calculate the factor by which the hydrogen ion concentration changes.
One common nitrogen-containing fertilizer is ammonium sulfate. State its chemical formula.
Markscheme
(i) Oxidation: loss of electrons and Reduction: gain of electrons;
(ii) As2O3: +3;
NO3–: +5;
H3AsO3: +3;
N2O3: +3;
Penalize incorrect notation e.g. III, V, 3+, 5+, 3, 5 once only.
(iii) Oxidizing agent: substance reduced / removes electrons from another substance / causes some other substance to be oxidized / OWTTE and Reducing agent: substance oxidized / gives electrons to another substance / causes some other substance to be reduced / OWTTE;
Accept Oxidizing agent: electron/e/e– acceptor / causes oxidation / oxidation number/state decreases and Reducing agent: electron/e/e– donor / causes reduction / oxidation number/state increases.
(iv) \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{2NO}}_3^ - {\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {\text{2}}{{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\)
correct coefficients for \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}\), \({{\text{H}}_3}{\text{As}}{{\text{O}}_4}\) and \({\text{NO}}_3^ - \), \({{\text{N}}_2}{{\text{O}}_3}\);
correct balanced equation;
Ignore state symbols.
M1 must be correct to award M2.
Oxidizing agent: \({\text{NO}}_3^ - {\text{(aq)}}\) / nitrate and Reducing agent: \({\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}}\) / arsenic(III) oxide;
Accept HNO3(aq)/nitric acid.
Accept arsenic oxide.
Species must be fully correct to score M3.
Ignore state symbols.
Brønsted Lowry theory: proton/H+ donor;
Lewis theory: electron-pair acceptor;
N=O;
accept any value in range 102–105°;
Actual value is 102°.
lone/non-bonding pairs on oxygen occupy more space/repel more than bonding pairs hence decreasing the H–O–N bond angle (from 109.5° ) / OWTTE;
Strong acid: acid/electrolyte completely/100% dissociated/ionized in solution/water / OWTTE and Weak acid: acid/electrolyte partially dissociated/ionized in solution/water / OWTTE;
 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
trigonal/triangular pyramidal;
Accept pyramidal (since SL).
Do not allow tetrahedral.
net dipole moment present in molecule / NH bond polarities do not cancel each other out / unsymmetrical distribution of charge /OWTTE;
Do not accept molecule has no symmetry hence polar.
changes by 102 /100;
Allow changes from 10–5 to 10–7.
\({{\text{(N}}{{\text{H}}_4}{\text{)}}_2}{\text{S}}{{\text{O}}_4}\);
Examiners report
This was the most popular question answered in Section B.
The definition of oxidation and reduction, deducing oxidation numbers (although some forgot the + sign) and distinguishing between an oxidizing and reducing agent was answered very well by a majority of the candidates. However, a surprising number of candidates were unable to balance the redox equation or identify the correct oxidizing and reducing agents in the given reaction.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
In part (b), most candidates defined an acid according to the Brønsted–Lowry and Lewis theories and identify the shorter bond in the Lewis structure given of \({\text{HN}}{{\text{O}}_{\text{2}}}\). Many candidates were able to deduce the approximate value of the H―O―N bond angle, however, some candidates were unable to explain in terms of the greater space occupied by the non-bonding electron pairs compared to the bonding electron pairs. Distinguishing between strong and weak acid in terms of their dissociation in aqueous solution was handled very well. The Lewis structure and shape of ammonia was done correctly by most candidates. However, the weaker candidates stated triangular planar instead of triangular pyramidal and that the molecule has no symmetry instead of unsymmetrical distribution of charge giving rise to a net dipole moment. The change in concentration with the change in pH was done well while an overwhelming number of candidates did not write the correct formula of ammonium sulphate.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a base according to both the Brønsted–Lowry and the Lewis theories of acids and bases.
The equation for the reaction between sodium hydroxide, NaOH, and nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}\), is shown below.
\[\begin{array}{*{20}{l}} {{\text{NaOH(aq)}} + {\text{HN}}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaN}}{{\text{O}}_3}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}}&{{\text{ }}\Delta H = - 57.6{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Distinguish between the terms strong base and weak base, and state one example of each.
State the equation for the reaction of ammonia with water.
Explain why ammonia can act as a Brønsted–Lowry base.
Explain why ammonia can also act as a Lewis base.
(i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is added to excess solid sodium carbonate, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}{\text{(s)}}\), an acid–base reaction occurs. Bubbles of gas are produced and the solid sodium carbonate decreases in mass. State one difference which would be observed if nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), was used instead of ammonium chloride.
(ii) Deduce the Lewis structures of the ammonium ion, \({\text{NH}}_4^ + \), and the carbonate ion, \({\text{CO}}_3^{2 - }\).
Ammonium ion\(\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \)Carbonate ion
(iii) Predict the shapes of \({\text{NH}}_4^ + \) and \({\text{CO}}_3^{2 - }\).
\({\text{NH}}_4^ + \):
\({\text{CO}}_3^{2 - }\):
(i) Sketch and label an enthalpy level diagram for this reaction.
(ii) Deduce whether the reactants or the products are more energetically stable, stating your reasoning.
(iii) Calculate the change in heat energy, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.50 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution is added to excess nitric acid.
When 5.35 g ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(s)}}\), is added to \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) of water, the temperature of the water decreases from 19.30 °C to 15.80 °C. Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the dissolving of ammonium chloride in water.
Markscheme
a strong base: base/electrolyte (assumed to be almost) completely/100% dissociated/ionized (in solution/water) / OWTTE and a weak base: base/electrolyte partially dissociated/ionized (in solution/water) / OWTTE;
example of a strong base: any group I hydroxide / \({\text{Ba(OH}}{{\text{)}}_2}\);
example of a weak base: \({\text{N}}{{\text{H}}_3}\) / \({\text{C}}{{\text{H}}_3}{\text{N}}{{\text{H}}_2}\) / any reasonable answer;
\({\text{N}}{{\text{H}}_3} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{NH}}_4^ + + {\text{O}}{{\text{H}}^ - }\);
accepts a proton/\({{\text{H}}^ + }\) / OWTTE;
donates an electron pair;
(i) more vigorous reaction / more gas bubbles / OWTTE;
more heat released;
solid decreases more quickly;
(ii) 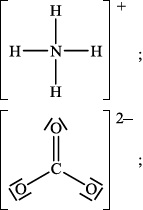
Accept any combination of lines, dots or crosses to represent electron pairs.
(iii) NH4+:
tetrahedral;
CO32–:
trigonal/triangular planar;
(i) enthalpy on y-axis;
Do not accept energy.
reactants higher than products;
\(\Delta H\) labelled;
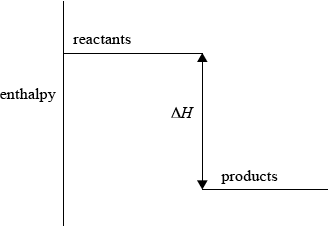
Accept appropriate formulas for reactants and products.
Arrow heads not needed.
57.6 is acceptable as an alternative to \(\Delta H\).
(ii) products are more stable as they are at a lower enthalpy level / energy has been given off by the reactants / reaction is exothermic / OWTTE;
(iii) \(n{\text{(NaOH)}} = 0.125{\text{ mol}}\);
change in heat energy \( = ( - 57.6 \times 0.125) = - 7.20{\text{ (kJ)}}\) / heat released \( = (57.6 \times 0.125) = 7.20{\text{ (kJ)}}\);
\(q = (mc\Delta T = ){\text{ }}100.0 \times 4.18 \times 3.50/1463{\text{ J}}/1460{\text{ J}}\);
\(n{\text{(N}}{{\text{H}}_{\text{4}}}{\text{Cl)}} = \frac{{5.35}}{{53.5}}/0.100{\text{ mol}}\);
\(\Delta H = + 14.6/14.6{\text{ (kJ mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept q = 105.35 \( \times \) 4.18 \( \times \) 3.50 / 1541 J.
Accept \(\Delta H\) = +15.4 / 15.4 (kJ\(\,\)mol–1)
Examiners report
Part (a) was answered well although some mentioned “dissolving” instead of “dissociating”.
In (b), the equation was well done.
In (b), the equation was well done as was (ii).
Inevitably, many omitted “pair” in (iii).
Part (c)(i) was generally correct. In (c)(ii) the carbonate ion was legitimately examined under AS 4.2.7; it was not well known – there were too many carbons with expanded octets and oxygens where the lone pairs had been missed. (In the HL specification, the carbonate ion‘s delocalization is considered.) In (iii), however, the shapes were well known.
If there was to be an error made in (d)(i), it was to omit “enthalpy” from the y-axis and some unaccountably put the correct chemicals on the line and then reversed the names products and reactants. The calculations in (d)(iii) inevitably depended on an ability to calculate and think logically.
The calculations in (e) inevitably depended on an ability to calculate and think logically.
Predict the shape and bond angles for the following species:
Ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), is a weak acid.
Draw the Lewis structures for carbon monoxide, CO, carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}\) and methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\).
List, with an explanation, the three compounds in order of increasing carbon to oxygen bond length (shortest first).
\({\text{C}}{{\text{O}}_{\text{2}}}\)
\({\text{CO}}_3^{2 - }\)
\({\text{BF}}_4^ - \)
Define a Brønsted-Lowry acid.
Deduce the two acids and their conjugate bases in the following reaction:
\[{{\text{H}}_2}{\text{O(l)}} + {\text{N}}{{\text{H}}_3}{\text{(aq)}} \rightleftharpoons {\text{O}}{{\text{H}}^ - }{\text{(aq)}} + {\text{NH}}_4^ + {\text{(aq)}}\]
Define the term weak acid and state the equation for the reaction of ethanoic acid with water.
Vinegar, which contains ethanoic acid, can be used to clean deposits of calcium carbonate from the elements of electric kettles. State the equation for the reaction of ethanoic acid with calcium carbonate.
Markscheme
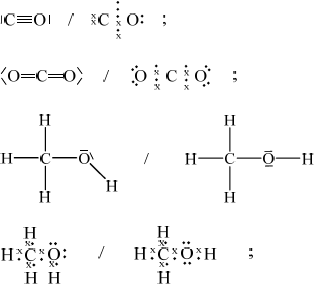
All outer electron pairs must be shown for mark in each case.
Accept electrons shown as all rather than \( \bullet \) and x.
\({\text{CO}} < {\text{C}}{{\text{O}}_{\text{2}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\);
triple bonds are shorter than double bonds which are shorter than single bonds / the more pairs of electrons that are shared the stronger the attracting so the shorter the bond / OWTTE;
The order must be correct to gain the second marking point unless ECF from (a).
\({\text{(C}}{{\text{O}}_{\text{2}}}{\text{)}}\)linear;
180°;
\({\text{(CO}}_3^{2 - }{\text{)}}\) trigonal planar/triangular planar;
120°;
\({\text{(BF}}_4^ - {\text{)}}\) tetrahedral;
109.5° / 109° / 109° \(28'\);
donates a proton / \({{\text{H}}^ + }\) ion;
\[\begin{array}{*{20}{c}} {{\text{(acid)}}}&{{\text{(conjugate base)}}} \\ {{{\text{H}}_{\text{2}}}{\text{O}}}&{{\text{O}}{{\text{H}}^ - }{\text{;}}} \\ {{\text{NH}}_4^ + }&{{\text{N}}{{\text{H}}_{\text{3}}}{\text{;}}} \end{array}\]
[1 max] if all four acids and bases given but not clearly paired.
partially dissociated or ionized;
\({\text{C}}{{\text{H}}_3}{\text{COOH}} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{H}}_3}{\text{COOH}} \rightleftharpoons {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}^ + }\);
\( \rightleftharpoons \) required for mark.
\({\text{2C}}{{\text{H}}_3}{\text{COOH}} + {\text{CaC}}{{\text{O}}_3} \to {\text{Ca(C}}{{\text{H}}_3}{\text{COO}}{{\text{)}}_2} + {\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}\)
Award [1] for correct reactants and products and [1] for balancing.
Examiners report
This was, by far, the most popular choice of question in Section B.
Part (a)(i) was well answered, though the weaker candidates often drew a double bond in carbon monoxide or missed out lone pairs.
These errors then gave rise to problems in attempting to answer (a)(ii).
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.
The better candidates scored all six marks for Part (b), the weaker candidates commonly giving the correct names more often than the correct angles.\[28'\]
In Part (c) the definition was generally well answered and the acids and bases were usually correctly identified though not always paired as asked for in the question.
In Part (c) the definition was generally well answered and the acids and bases were usually correctly identified though not always paired as asked for in the question.
In the final equation it was rare to see a correct formula for calcium ethanoate, and even when present, the equation was not usually balanced.
In the final equation it was rare to see a correct formula for calcium ethanoate, and even when present, the equation was not usually balanced.
Phosphine (IUPAC name phosphane) is a hydride of phosphorus, with the formula PH3.
(i) Draw a Lewis (electron dot) structure of phosphine.
(ii) Outline whether you expect the bonds in phosphine to be polar or non-polar, giving a brief reason.
(iii) Explain why the phosphine molecule is not planar.
(iv) Phosphine has a much greater molar mass than ammonia. Explain why phosphine has a significantly lower boiling point than ammonia.
Phosphine is usually prepared by heating white phosphorus, one of the allotropes of phosphorus, with concentrated aqueous sodium hydroxide. The equation for the reaction is:
P4 (s) + 3OH− (aq) + 3H2O (l) → PH3 (g) + 3H2PO2− (aq)
(i) Identify one other element that has allotropes and list two of its allotropes.
Element:
Allotrope 1:
Allotrope 2:
(ii) The first reagent is written as P4, not 4P. Describe the difference between P4 and 4P.
(iii) The ion H2PO2− is amphiprotic. Outline what is meant by amphiprotic, giving the formulas of both species it is converted to when it behaves in this manner.
(iv) State the oxidation state of phosphorus in P4 and H2PO2−.
P4:
H2PO2−:
(v) Oxidation is now defined in terms of change of oxidation number. Explore how earlier definitions of oxidation and reduction may have led to conflicting answers for the conversion of P4 to H2PO2− and the way in which the use of oxidation numbers has resolved this.
2.478 g of white phosphorus was used to make phosphine according to the equation:
P4(s) +3OH−(aq)+3H2O(l) → PH3(g)+3H2PO2−(aq)
(i) Calculate the amount, in mol, of white phosphorus used.
(ii) This phosphorus was reacted with 100.0 cm3 of 5.00 mol dm−3 aqueous sodium hydroxide. Deduce, showing your working, which was the limiting reagent.
(iii) Determine the excess amount, in mol, of the other reagent.
(iv) Determine the volume of phosphine, measured in cm3 at standard temperature and pressure, that was produced.
Markscheme
(i)
Accept structures using dots and/or crosses to indicate bonds and/or lone pair.
(ii)
non-polar AND P and H have the same electronegativity
Accept “similar electronegativities”.
Accept “polar” if there is a reference to a small difference in electronegativity and apply ECF in 1 a (iv).
(iii)
4 electron domains/pairs/negative charge centres «around the central atom»
OR
a lone/non-bonding pair «and three bonding pairs around the central atom»
repulsion between electron domains/pairs/negative charge centres «produces non-planar shape»
OR
«repulsion causes» tetrahedral orientation/pyramidal shape
(iv)
PH3 has London «dispersion» forces
NH3 forms H-bonds
H-bonds are stronger
OR
London forces are weaker
Accept van der Waals’ forces, dispersion forces and instantaneous dipole – induced dipole forces.
Accept “dipole-dipole forces” as molecule is polar.
H-bonds in NH3 (only) must be mentioned to score [2].
Do not award M2 or M3 if:
• implies covalent bond is the H-bond
• implies covalent bonds break.
Accept “dipole-dipole forces are weaker”.
(i)
Element
carbon/C
OR
oxygen/O/O2
Allotropes
Award [1] for two of:
diamond
graphite
graphene
C60 / buckminsterfullerene
OR
ozone/O3 AND «diatomic/molecular» oxygen/O2
Accept two correctly named allotropes of any other named element (S, Se, Sn, As, etc.).
Accept fullerene, “buckyballs” etc. instead of buckminsterfullerene.
(ii)
P4 is a molecule «comprising 4P atoms» AND 4P is four/separate «P» atoms
OR
P4 represents «4P» atoms bonded together AND 4P represents «4» separate/non-bonded «P» atoms
(iii)
can act as both a «Brønsted–Lowry» acid and a «Brønsted–Lowry» base
OR
can accept and/or donate a hydrogen ion/proton/H+
HPO22− AND H3PO2
(iv)H2PO2− : +1
OR
negative charge «on product/H2PO2− » /gain of electrons so could be reduction
Do not award M3 for “oxidation number changes”.
(i)
«\(\left\langle {\frac{{2.478}}{{4 \times 30.97}}} \right\rangle \)»= 0.02000«mol»
(ii)
n(NaOH)=«0.1000×5.00=»0.500«mol» AND P4/phosphorus is limiting reagent
Accept n(H2O) =\(\frac{{100}}{{18}}\) = 5.50 AND P4 is limiting reagent.
(iii)
amount in excess «= 0.500 - (3 × 0.02000)» = 0.440 «mol»
(iv)
«22.7 × 1000 × 0.02000» = 454 «cm3»
Accept methods employing pV = nRT, with p as either 100 (454 cm3) or 101.3 kPa (448 cm3).
Do not accept answers in dm3.
Examiners report
Acids play a key role in processes in everyday life.
The wine industry is important to the economy of many countries. Wine contains ethanol. In a laboratory in Chile, chemists tested the pH of a bottle of wine when opened and found it to have a pH of 3.8. After a few days, the pH had decreased to 2.8.
Deduce the change in hydrogen ion concentration, \({\text{[}}{{\text{H}}^ + }{\text{]}}\).
State the name of the compound formed that is responsible for this decreased pH value.
Sulfuric acid present in acid rain can damage buildings made of limestone. Predict the balanced chemical equation for the reaction between limestone and sulfuric acid including state symbols.
Markscheme
\({\text{[}}{{\text{H}}^ + }{\text{]}}\) increased by factor of 10;
Allow a difference of 1.426 \( \times \) 10–3.
ethanoic acid;
Allow acetic acid.
\({\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {\text{CaS}}{{\text{O}}_4}{\text{(s)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\)
correct chemical equation;
correct state symbols;
Allow CaSO4(aq) instead of CaSO4(s).
M2 can only be scored if M1 is correct.
Award [1max] if H2CO3(aq) is given instead of H2O(l) + CO2(g).
Examiners report
Question 3 a)(i) presented difficulties to some candidates who attempted to calculate the concentration of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) ions even though this is not on the SL course. Simply recognizing that a decrease in pH of 1 unit is equivalent to an increase in \({\text{[}}{{\text{H}}^ + }{\text{]}}\) by a factor of 10 was sufficient here (A.S. 8.4.3).
In a) (ii) many candidates correctly identified ethanoic acid as the cause of the decrease in pH. Some simply stated carboxylic acid, which is a class of compound and not a name of a compound.
Part b) was a challenge to candidates who did not know the formula of limestone. This reaction is mentioned in teachers’ notes in 8.3.1. State symbols were also required. Some candidates mistakenly identified sulfuric acid in acid rain as H2SO4(l) and did not score the second mark.
Aspirin, one of the most widely used drugs in the world, can be prepared according to the equation given below.
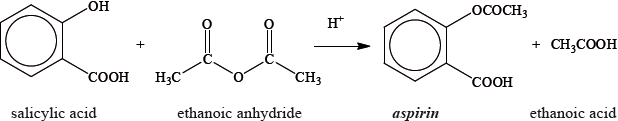
A student reacted some salicylic acid with excess ethanoic anhydride. Impure solid aspirin was obtained by filtering the reaction mixture. Pure aspirin was obtained by recrystallization. The following table shows the data recorded by the student.

State the names of the three organic functional groups in aspirin.
Determine the amount, in mol, of salicylic acid, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OH)COOH}}\), used.
Calculate the theoretical yield, in g, of aspirin, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OCOC}}{{\text{H}}_{\text{3}}}{\text{)COOH}}\).
Determine the percentage yield of pure aspirin.
State the number of significant figures associated with the mass of pure aspirin obtained, and calculate the percentage uncertainty associated with this mass.
Another student repeated the experiment and obtained an experimental yield of 150%. The teacher checked the calculations and found no errors. Comment on the result.
The following is a three-dimensional computer-generated representation of aspirin.
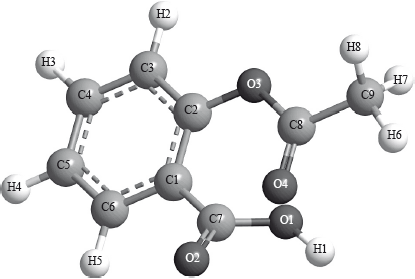
A third student measured selected bond lengths in aspirin, using this computer program and reported the following data.
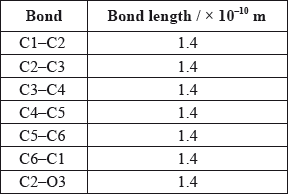
The following hypothesis was suggested by the student: “Since all the measured carbon-carbon bond lengths are equal, all the carbon-oxygen bond lengths must also be equal in aspirin. Therefore, the C8–O4 bond length must be 1.4 \( \times \) 10–10 m”. Comment on whether or not this is a valid hypothesis.
The other product of the reaction is ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\). Define an acid according to the Brønsted-Lowry theory and state the conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\).
Brønsted-Lowry definition of an acid:
Conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\):
Markscheme
carboxylic acid / carboxyl;
ester;
Do not allow carbonyl / acid / ethanoate / formula(–COOH).
aryl group / benzene ring / phenyl;
\({M_{\text{r}}}{\text{(}}{{\text{C}}_7}{{\text{H}}_6}{{\text{O}}_3}{\text{)}} = {\text{138.13}}\);
\(n = \left( {\frac{{3.15}}{{138.13}} = } \right){\text{ }}2.28 \times {10^{ - 2}}{\text{ (mol)}}\);
Award [2] for the correct final answer.
\({M_{\text{r}}}{\text{(}}{{\text{C}}_9}{{\text{H}}_8}{{\text{O}}_4}{\text{)}} = 180.17\);
\(m = (180.17 \times 2.28 \times {10^{ - 2}} = ){\text{ }}4.11{\text{ (g)}}\);
Accept range 4.10–4.14
Award [2] for the correct final answer.
\({\text{(percentage yield}} = \frac{{2.50}}{{4.11}} \times 100 = ){\text{ }}60.8\% \);
Accept 60–61%.
3;
\({\text{(percentage uncertainty }} = \frac{{0.02}}{{2.50}} \times 100 = {\text{) }}0.80\% \);
Allow 0.8%
sample contaminated with ethanoic acid / aspirin not dry / impure sample;
Accept specific example of a systematic error.
Do not accept error in reading balance/weighing scale.
Do not accept yield greater than 100%.
hypothesis not valid/incorrect;
Accept any of the following for the second mark
C–O and C=O bond lengths will be different;
C2–O3 bond is longer than C8–O4 bond;
C8–O4 bond shorter than C2–O3 bond;
a CO single bond is longer than a CO double bond;
Accept C8–O4 is a double bond hence shorter.
Brønsted-Lowry definition of an acid
proton/H+/hydrogen ion donor;
Conjugate base of CH3COOH
\({\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{/C}}{{\text{H}}_3}{\text{CO}}_2^ - \);
Do not accept C2H3O2–/ethanoate.
Examiners report
In (a) Some candidates gave the correct three names of the functional groups; however some candidates gave answers such as alkene, ketone, aldehyde, ether, and carbonyl.
Candidates did not have problems determining the number of moles of salicylic acid used in (b) (i), although a few gave the answer with one significant digit only.
For (ii) the majority of candidates correctly used the value obtained in (i) to calculate the theoretical yield of aspirin.
In (iii) the percentage yield was calculated correctly in most cases.
The calculation of the percentage uncertainty (part (iv) proved to be a little more difficult, but many candidates gave the correct answer of 0.80%.
Part (v) was correctly answered by only a few candidates who stated that aspirin was contaminated or that the aspirin was not dry.
Nearly all the candidates correctly stated that the suggested hypothesis was not valid in (vi), giving the right reasons.
In (vii) most candidates gave the correct definition of an acid according to Brønsted-Lowry theory, although a few defined the acid according to Lewis theory. The conjugate base of the ethanoic acid was not always correct.
The boiling points of the isomers of pentane, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\), shown are 10, 28 and 36 °C, but not necessarily in that order.
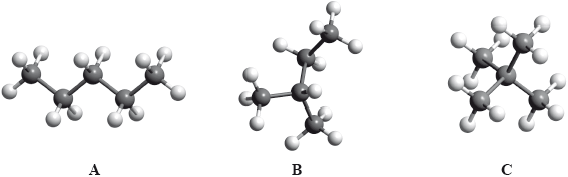
Identify the boiling points for each of the isomers A, B and C and state a reason for your answer.

State the IUPAC names of isomers B and C.
B:
C:
Both \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) and \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) can be used as fuels. Predict which compound would release a greater amount of heat per gram when it undergoes complete combustion. Suggest two reasons to support your prediction.
In many cities around the world, public transport vehicles use diesel, a liquid hydrocarbon fuel, which often contains sulfur impurities and undergoes incomplete combustion. All public transport vehicles in New Delhi, India, have been converted to use compressed natural gas (CNG) as fuel. Suggest two ways in which this improves air quality, giving a reason for your answer.
Markscheme
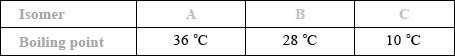
Award [1] if correct boiling points are assigned to 3 isomers.
increase in branching / more side chains / more spherical shape / reduced surface contact / less closely packed;
weaker intermolecular force/van der Waals’/London/dispersion forces;
Accept the opposite arguments
B: 2-methylbutane/methylbutane;
C: 2,2-dimethyl propane/dimethyl propane;
Do not penalize missing commas, hyphens or added spaces.
Do not accept 2-dimethylpropane, or 2,2-methylpropane.
\({{\text{C}}_5}{{\text{H}}_{12}}\);
Accept any two of the following explanations.
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) has greater molar mass / produces less grams of \({\text{C}}{{\text{O}}_2}\) and \({{\text{H}}_2}{\text{O}}\) per gram of the compound / suitable calculations to show this;
\({{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}}\) contains an O atom which contributes nothing to the energy released / partially oxidized / OWTTE;
analogous compounds such as butane and butan-1-ol show a lower value for the alcohol per mole in the data book / OWTTE;
the total bond strength in the pentanol molecule is higher than the total bond strength in pentane;
the total amount of energy produced in bond formation of the products per mole is the same;
fewer moles of pentanol in 1 g;
pentanol requires more energy to break intermolecular forces/hydrogen bonding / OWTTE;
Improvements [2]
less/no particulates/C/CO/VOC’s produced with CNG;
less/no SO2/SOx produced;
Reasons [1 max]
CO/SO2 toxic/poisonous;
SO2 causes acid rain;
CNG is likely to undergo complete/more combustion;
CNG has no/less sulfur impurities;
Examiners report
This question also featured on the G2 forms, as some teachers thought that the inclusion of Aim 8 type questions such as this would disadvantage candidates. However performance by the majority was very good. It should be noted that questions of this type will always be asked in future papers. In (a), most candidates correctly identified the boiling points although some reversed the order and a few had B with the highest boiling point. Explanations for this trend were not so well answered. Some candidates referred to breaking bonds in the carbon chain and several answers referred to the length of the carbon chain rather than the degree of branching.
The IUPAC names were generally well known, with the most common errors being the use of “pent” instead of “prop” and the omission of one of the locants, or “di” in “2,2-dimethylpropane”.
Many candidates scored 0 in part b) as they incorrectly suggested that pentan-1-ol would have a larger energy density than pentane. It is clear from the variety of wrong answers and reasons that candidates are not familiar with the ideas tested in this question. Many candidates referred to hydrogen bonds between molecules, as a reason for pentan-1-ol releasing more energy, only a few consulted their Data Booklet and made reference to this.
In c) there were 2 marks for improvements to air quality and 1 mark for a reason. Most candidates included the idea that there would be less carbon monoxide formed and that this was a poisonous gas. There were fewer references to oxides of sulfur, although many said that CNG has fewer S impurities rather than to say that less SO2/SOx is released, in this case as they had already scored their explanation mark they could not score for this and ended up with 2 marks out of 3. Some candidates did not centre their answer on what was being asked. Also, some candidates said that natural gas is a natural fuel while diesel is not, and that natural gas, when it burns does not produce carbon dioxide.
In acidic solution, ions containing titanium can react according to the half-equation below.
\[{\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
A reactivity series comparing titanium, cadmium and europium is given below.
Least reactive Cd \( < \) Ti \( < \) Eu Most reactive
The half-equations corresponding to these metals are:
\({\text{E}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Eu(s)}}\)
\({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Ti(s)}}\)
\({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Cd(s)}}\)
Some students were provided with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a monobasic acid, HQ, and given the problem of determining whether HQ was a weak acid or a strong acid.
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
Deduce which of the species would react with titanium metal.
Deduce the balanced equation for this reaction.
Deduce which of the six species is the strongest oxidizing agent.
A voltaic cell can be constructed using cadmium and europium half-cells. State how the two solutions involved should be connected and outline how this connection works.
Define a Brønsted–Lowry acid.
Distinguish between the terms strong acid and weak acid.
Neelu and Charles decided to solve the problem by determining the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution needed to neutralize \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of the acid. Outline whether this was a good choice.
Neelu and Charles decided to compare the volume of sodium hydroxide solution needed with those required by known \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) strong and weak acids. Unfortunately they chose sulfuric acid as the strong acid. Outline why this was an unsuitable choice.
State a suitable choice for both the strong acid and the weak acid.
Strong acid:
Weak acid:
Francisco and Shamiso decided to measure the pH of the initial solution, HQ, and they found that its pH was 3.7. Deduce, giving a reason, the strength (weak or strong) of the acid HQ.
Suggest a method, other than those mentioned above, that could be used to solve the problem and outline how the results would distinguish between a strong acid and a weak acid.
Markscheme

+ sign must be present. Do not award mark for incorrect notation 4, 4+, 3, 3+ etc.
Do not award M2 if inconsistent with M1.
increases / makes it stronger;
(more \({{\text{H}}^ + }\) would) drive/shift equilibrium to the right/towards products
(accepting more electrons);
\({\text{C}}{{\text{d}}^{2 + }}\);
Do not allow incorrect notation such as Cd, Cd(II), or Cd+2.
\({\text{2Ti(s)}} + {\text{3C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{2T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3Cd(s)}}\);
Ignore state symbols.
Allow ECF from (b)(i) for a correct equation.
\({\text{C}}{{\text{d}}^{2 + }}\);
Charge must be given.
Do not allow incorrect notation such as Cd, Cd(II), or Cd+2 but penalize
only once in b(i) and b(iii) .
Allow ECF, if Eu2+ is written both in part (i) and part (iii).
salt bridge;
Accept specific examples of salt bridges, such as filter paper dipped in aqueous KNO3.
allows the movement of ions (between the two solutions) / completes the circuit / maintains electrical neutrality;
Accept movement of charges/negative ions/positive ions.
donates \({{\text{H}}^ + }\)/protons;
strong acid completely/100%/fully dissociated/ionized and weak acid partially/slightly dissociated/ionized;
not a good choice / poor choice;
requires same volume of the base / the amount/volume to react/for neutralization does not depend on the acid strength;
sulfuric acid is diprotic/dibasic/liberates two protons/\({{\text{H}}^ + }\);
Accept “reacts with 2 moles of alkali/base”.
Strong acid: hydrochloric acid/HCl / nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\);
Weak acid: ethanoic acid/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\);
Allow acetic acid for weak acid.
Accept any other strong/weak monobasic acids as appropriate.
Do not accept non-monobasic acids, such as phosphoric acid and carbonic acid.
weak;
strong \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) acid has a pH of 1/lower than that observed;
Accept “pH value of 3.7 means that it produces only 10–3.7/2.0 \( \times \) 10–4 [H+] in water”.
measure the rate of reaction with reactive metal/(metal) carbonate/metal oxide;
strong acid would react faster/more vigorously / weak acid would react slower/less vigorously;
Accept specific substances, such as Mg and CaCO3, which react with acids.
OR
measure conductivity;
higher for strong acid / lower for weak acid;
OR
measure heat/enthalpy of neutralization;
greater for strong acid / lower for weak acid;
Do not accept pH/universal indicator paper.
Examiners report
In part (a) (i), most candidates scored full marks although some candidates continue to write incorrect notation (4, 4+) for oxidation states.
In part (ii), some candidates missed the word equilibrium in the question and hence could not state that equilibrium will shift towards right and strength of oxidizing agent will increase.
In part (b) (i), (iii), the correct answer was \({\text{C}}{{\text{d}}^{2 + }}\) but many candidates wrote Cd, Eu or Ti.
In part (ii), the better candidates wrote the correct balanced chemical equation. Some included electrons in the equation which was surprising and some did not read the question where the reaction with Ti metal was asked.
In part (b) (i), (iii), the correct answer was \({\text{C}}{{\text{d}}^{2 + }}\) but many candidates wrote Cd, Eu or Ti.
In part (iv), many candidates identified the salt bridge but some missed the reference to the movement of ions.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
In part (c), most candidates were able to define a Bronsted-Lowry acid. The difference between strong and weak was usually correctly stated although only better candidates stated that strong acid is assumed to be 100% dissociated. Part (iii) proved to be difficult where very few candidates stated correctly that it is not a good choice because it requires the same volume of the base. Many candidates, however, knew the fact that sulfuric acid is diprotic in part (iv). In part (v), majority of candidates correctly identified the strong and weak acid whereas weaker candidates stated NaOH as a weak acid. Part (vi) was poorly done with many candidates stating pH 3.7 as strong acid. In part (vii), many candidates scored full marks but universal indicator paper was often suggested, which of course, scored no marks.
Some of the most important processes in chemistry involve acid-base reactions.
Describe the acid-base character of the oxides of each of the period 3 elements, Na to Cl.
State one example of an acidic gas, produced by an industrial process or the internal combustion engine, which can cause large-scale pollution to lakes and forests.
Suggest one method, other than measuring pH, which could be used to distinguish between solutions of a strong acid and a weak acid of the same molar concentration. State the expected results.
Markscheme
Na, Mg: basic;
Al: amphoteric;
Do not accept amphiprotic.
Si to Cl: acidic;
Award [1] for stating oxides become more basic towards left/Na and more acidic towards right/Cl.
Do not penalize incorrect formulas of oxides.
\({\text{N}}{{\text{O}}_{\text{2}}}\)/nitrogen dioxide / \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\)/dinitrogen tetroxide / \({\text{S}}{{\text{O}}_{\text{2}}}\)/sulfur dioxide / \({\text{S}}{{\text{O}}_{\text{3}}}\)/sulfur trioxide;
Do not accept NO/NOx/CO2/CO.
measure electrical conductivity;
strong acids are good conductors/better conductors than weak acids / weak acids are poor conductors;
OR
react with magnesium or a named active metal/metal carbonate/hydrogen carbonate/bicarbonate;
Do not accept Na/K
strong acids react faster/more gas bubbles (per unit time)/more heat produced / weak acids react slower/less gas bubbles (per unit time)/less heat produced;
Do not accept answers based on titration curves as they are based on pH.
Accept Neutralization: weak acid would produce less energy/less temperature increase compared to a strong acid.
Examiners report
The majority of candidates gave the correct answers to (a), but a few were confused about the acid-base character of the oxides of aluminium and silicon.
Part (b) proved to be a difficult question. Not many candidates gave the name or formula of an acidic gas produced by an industrial process. Some wrong answers were: CO, SO, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\), CFCs, Methane, \({\text{N}}{{\text{H}}_{\text{3}}}\).
There were a few good answers to (c); measuring the conductivity or the reaction with magnesium or calcium carbonate was a possible method for distinguishing between a strong and a weak acid of the same concentration.
0.100 g of magnesium ribbon is added to \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid to produce hydrogen gas and magnesium sulfate.
\[{\text{Mg(s)}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} \to {{\text{H}}_2}{\text{(g)}} + {\text{MgS}}{{\text{O}}_4}{\text{(aq)}}\]
Magnesium sulfate can exist in either the hydrated form or in the anhydrous form. Two students wished to determine the enthalpy of hydration of anhydrous magnesium sulfate. They measured the initial and the highest temperature reached when anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}{\text{(s)}}\), was dissolved in water. They presented their results in the following table.

The students repeated the experiment using 6.16 g of solid hydrated magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}} \bullet {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(s)}}\), and \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. They found the enthalpy change, \(\Delta {H_2}\), to be \( + 18{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
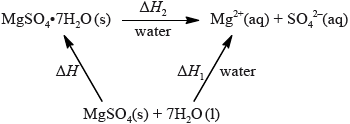
The enthalpy of hydration of solid anhydrous magnesium sulfate is difficult to determine experimentally, but can be determined using the diagram below.
Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic limestone. This limestone is a mixture of magnesium carbonate and calcium carbonate.
(i) The graph shows the volume of hydrogen produced against time under these experimental conditions.
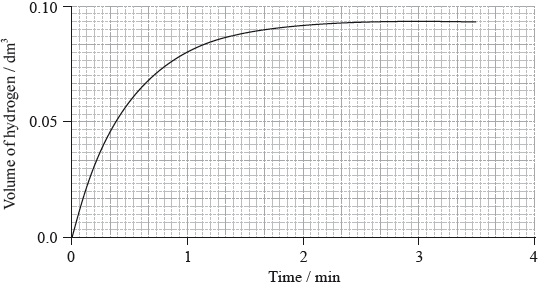
Sketch two curves, labelled I and II, to show how the volume of hydrogen produced (under the same temperature and pressure) changes with time when:
I. using the same mass of magnesium powder instead of a piece of magnesium ribbon;
II. 0.100 g of magnesium ribbon is added to \({\text{50 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid.
(ii) Outline why it is better to measure the volume of hydrogen produced against time rather than the loss of mass of reactants against time.
(i) Calculate the amount, in mol, of anhydrous magnesium sulfate.
(ii) Calculate the enthalpy change, \(\Delta {H_1}\), for anhydrous magnesium sulfate dissolving in water, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). State your answer to the correct number of significant figures.
(i) Determine the enthalpy change, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the hydration of solid anhydrous magnesium sulfate, \({\text{MgS}}{{\text{O}}_{\text{4}}}\).
(ii) The literature value for the enthalpy of hydration of anhydrous magnesium sulfate is \( - 103{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage difference between the literature value and the value determined from experimental results, giving your answer to one decimal place. (If you did not obtain an answer for the experimental value in (c)(i) then use the value of \( - 100{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the correct value.)
Another group of students experimentally determined an enthalpy of hydration of \( - 95{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Outline two reasons which may explain the variation between the experimental and literature values.
(i) State the equation for the reaction of sulfuric acid with magnesium carbonate.
(ii) Deduce the Lewis (electron dot) structure of the carbonate ion, giving the shape and the oxygen-carbon-oxygen bond angle.
Lewis (electron dot) structure:
Shape:
Bond angle:
Markscheme
(i) 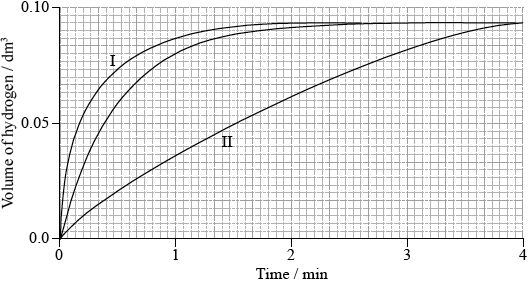
I: line which is steeper/increases faster and finishes at the same height;
II: line which is less steep/increases more slowly and finishes at the same height;
(ii) mass of hydrogen produced is very small (so not accurate) / decrease in mass is very small (so not accurate);
(i) \(n({\text{MgS}}{{\text{O}}_4}) = \left( {\frac{{3.01}}{{120.37}} = } \right){\text{ }}0.0250{\text{ (mol)}}\);
(ii) energy released \( = 50.0 \times 4.18 \times 9.7 \times 2027{\text{ (J)}}/2.027{\text{ (kJ)}}\);
\(\Delta {H_1} = - 81{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [2] for correct answer.
Award [2] if 53.01 is used giving an answer of –86 (kJ mol–1).
Award [1 max] for +81/81/+86/86 (kJ mol−1).
Award [1 max] for –81000/–86000 if units are stated as J mol−1.
Allow answers to 3 significant figures.
(i) \(\Delta H{\text{ }}( = \Delta {H_1} - \Delta {H_2}) = - 99{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [1] if –86 is used giving an answer of –104 (kJ mol−1).
(ii) \(\frac{{(103 - 99)}}{{103}} \times 100 = 3.9\% \);
Accept answer of 2.9 % if –100 used but only if a value for (b)(i) is not
present.
Award [1] if –104 is used giving an answer of 1.0% .
Accept correct answers which are not to 1 decimal place.
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) not completely anhydrous / OWTTE;
\({\text{MgS}}{{\text{O}}_{\text{4}}}\) is impure;
heat loss to the atmosphere/surroundings;
specific heat capacity of solution is taken as that of pure water;
experiment was done once only so it is not scientific;
density of solution is taken to be \(1{\text{ g}}\,{\text{c}}{{\text{m}}^{ - 3}}\);
mass of \(7{{\text{H}}_2}{\text{O}}\) ignored in calculation;
uncertainty of thermometer is high so temperature change is unreliable;
literature values determined under standard conditions but this experiment is not;
all solid not dissolved;
(i) \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}} + {\text{MgC}}{{\text{O}}_3}{\text{(s)}} \to {\text{MgS}}{{\text{O}}_4}{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
Do not accept H2CO3.
(ii) 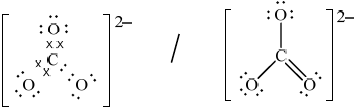 ;
;
Accept crosses, lines or dots as electron pairs.
Accept any correct resonance structure.
Award [0] if structure is drawn without brackets and charge.
Award [0] if lone pairs not shown on O atoms.
shape: trigonal/triangular planar;
bond angle: 120°;
Accept answers trigonal/triangular planar and 120° if M1 incorrect, but no other answer should be given credit.
Examiners report
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
Many candidates could sketch correct curves in (a)(i), though many did not realize that the same final volume of hydrogen is formed. Lines were generally poorly drawn with several lines for one curve, and curve I often did not join smoothly with the given curve, but dropped near the end or overshot the final volume and then fell back down. Candidates are advised to draw graphs in pencil first. In (a)(ii), very few students indicated that because the mass of hydrogen is very small it is better to measure reaction rate using gas volume; most indicated that it is not precise because the mass of a mixture is measured. It seems that very few candidates are aware that measuring loss of mass per unit time is a valid tool for determining the rate of a reaction when \({\text{C}}{{\text{O}}_{\text{2}}}\) is produced. The moles of magnesium sulfate were mostly calculated correctly in (b)(i), but in (b)(ii) most candidates had problems calculating the enthalpy change, working with the mass of magnesium sulfate instead of water or solution and not giving the enthalpy change a negative sign. Several candidates only found the temperature change and called this the enthalpy change, or found the energy change and ignored the number of moles. Few candidates correctly applied Hess’s law in (c)(i). Some respondents felt that this was not on the SL course, but it is clearly stated in 5.3.1. Some candidates had no idea how to calculate the percentage difference in (c)(ii) and several left this blank despite a value being given for the experimental results for candidates to use if they had not found a value themselves. Quite a few others determined the percentage difference correctly. In (d) most candidates stated heat loss to the surroundings as an error, mentioning further irrelevant errors. Only the better candidates also referred to the partial hydration of the anhydrous salt. The equation for the reaction between sulfuric acid and magnesium carbonate was generally done well in (e)(i) but \({{\text{H}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\) was frequently (incorrectly) given as a product. A few candidates did not know the formulas for sulfuric acid and magnesium carbonate. Very few candidates could give a correct Lewis structure for the carbonate ion in (ii). Some almost scored but failed to include brackets and charge. Some decided that the carbonate ion was a synonym for carbon dioxide and drew that. The formula for the carbonate ion should be known (assessment statement 4.1.7) and only one Lewis structure was required so students did not need to know about resonance structures. Shape and bond angle were also done poorly but there were a few candidates who knew the shape and bond angle of the carbonate ion even though they couldn’t draw the Lewis structure.
A sample of magnesium contains three isotopes: magnesium-24, magnesium-25 and magnesium-26, with abundances of 77.44%, 10.00% and 12.56% respectively.
Phosphorus(V) oxide, \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{ }}({M_{\text{r}}} = 283.88)\), reacts vigorously with water \(({M_{\text{r}}} = 18.02)\), according to the equation below.
\[{{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{(s)}} + {\text{6}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4}}{{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\]
Calculate the relative atomic mass of this sample of magnesium correct to two decimal places.
Predict the relative atomic radii of the three magnesium isotopes, giving your reasons.
Describe the bonding in magnesium.
State an equation for the reaction of magnesium oxide with water.
A student added 5.00 g of \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) to 1.50 g of water. Determine the limiting reactant, showing your working.
Calculate the mass of phosphoric(V) acid, \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\), formed in the reaction.
State a balanced equation for the reaction of aqueous \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\) with excess aqueous sodium hydroxide, including state symbols.
State the formula of the conjugate base of \({{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{4}}}\).
(i) Deduce the Lewis structure of \({\text{PH}}_4^ + \).
(ii) Predict, giving a reason, the bond angle around the phosphorus atom in \({\text{PH}}_4^ + \).
(iii) Predict whether or not the P–H bond is polar, giving a reason for your choice.
Markscheme
\(\left( {\frac{{(77.44 \times 24) + (10.00 \times 25) + (12.56{\text{ }}26)}}{{100}}} \right)\);
24.35;
Award [2] for correct final answer.
Two decimal places are required for M2.
Do not award any marks for 24.31 without showing method (as the value can be copied from the Data Booklet).
same atomic radii / 160 pm;
isotopes only differ by number of neutrons/size of nucleus / radius
determined by electron shells and number of protons / OWTTE;
Accept neutrons do not affect distance of electrons / OWTTE.
(lattice of) positive ions/cations and mobile/free/delocalized electrons;
Accept “sea of electrons” instead of “delocalized electrons”.
Award M1 for a suitable diagram.
electrostatic attraction (between ions and delocalized electrons);
\({\text{MgO}} + {{\text{H}}_{\text{2}}}{\text{O}} \to {\text{Mg(OH}}{{\text{)}}_{\text{2}}}/{\text{M}}{{\text{g}}^{2 + }} + {\text{2O}}{{\text{H}}^ - }\);
Accept reversible arrow.
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{: }}\left( {\frac{{{\text{5.00}}}}{{{\text{283.88}}}} = } \right){\text{ 0.0176 (mol)}}\) and \({{\text{H}}_2}{\text{O: }}\left( {\frac{{{\text{1.50}}}}{{{\text{18.02}}}} = } \right){\text{ 0.0832 (mol)}}\);
\({{\text{H}}_2}{\text{O}}\) is the limiting reactant and reason related to stoichiometry;
\(\frac{{0.0832 \times 4}}{6}/0.0555{\text{ (mol)}}\);
\((0.0555 \times 98.00 = ){\text{ }}5.44{\text{ g}}\);
The unit is needed for M2.
Award [2] for correct final answer.
Do not penalize slight numerical variations due to premature rounding.
\({{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3NaOH(aq)}} \to {\text{N}}{{\text{a}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\)
correct products and balancing;
correct state symbols;
Accept valid ionic equations.
\({{\text{H}}_2}{\text{PO}}_4^ - \);
(i)  ;
;
Accept dots, crosses or lines for pairs of electrons.
No need to distinguish the dative covalent bond from the other bonds.
Charge is required for the mark.
Do not penalize missing square brackets.
(ii) \(109^\circ 27'/109.5^\circ /109^\circ \);
4 electron domains/pairs/(negative) charge centres (around central atom/P);
Accept ion is tetrahedral / electron pairs/domains repel each other.
(iii) non-polar and P and H have the same electronegativity / OWTTE;
Accept slightly polar as precise electronegativities of P and H are not identical / OWTTE.
Examiners report
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
In Part (a) most candidates gained full marks, with the most common error being a failure to quote the answer to the precision specified, but the explanations of deflection, and more particularly detection, in the mass spectrometer were weak. The prediction of relative atomic radii of the isotopes, something that required the application of reason rather than recall, also proved much more challenging. Part (b) revealed that many candidates have a very weak understanding of the metallic bond with many thinking the bonding was ionic.
Even when they knew about a cation lattice and delocalized electrons, a mark was frequently dropped by failing to specify that the attraction between them was electrostatic. Most candidates wrote the correct equation in Part (c), but it is still disturbing that some students at this level cannot write even the most straightforward chemical equation. In Part (d) many students proved capable of carrying out routine stoichiometric calculations to identify the limiting reactant and use the result to find the mass of the product.
Even if the final result was incorrect quite frequently students gained some credit through the application of ECF. Only the better candidates could write an equation for the neutralisation of phosphoric(V) acid and even the routine derivation of a conjugate base from the formula of the acid proved difficult for many. In Part (e) most students could manage the correct Lewis structure, though some lost the mark through omitting the charge. Many candidates also scored well on the shape of the ion and the polarity of the P-H bond.
A student used a pH meter to measure the pH of different samples of water at 298 K.
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
Markscheme
river (water);
\(\left( {\frac{{0.1}}{{5.1}} \times 100 = } \right){\text{ }}2\% \);
recognition that values differ by 2 Ph units / calculation of both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values;
\({\text{(ratio)}} = 1:100/{10^{ - 2}}/0.01/\frac{1}{{100}}\);
Award [2] for correct final answer.
Award [1 max] for 100:1/100/102.
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}^ + }/{\text{C}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{H}}_2}{\text{C}}{{\text{O}}_3}\);
Do not penalize missing reversible arrow.
Do not accept equations with the carbonate ion as a product.
Examiners report
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
The equations of two acid-base reactions are given below.
Reaction A \({\text{N}}{{\text{H}}_{\text{3}}}({\text{aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons \) \({\rm{NH}}_4^ + ({\rm{aq}}) + {\rm{O}}{{\rm{H}}^ - }({\rm{aq}})\)
The reaction mixture in A consists mainly of reactants because the equilibrium lies to the left.
Reaction B \({\text{NH}}_2^ -({\text{aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons \) \({\rm{NH}}_3^{}({\rm{aq}}) + {\rm{O}}{{\rm{H}}^ - }({\rm{aq}})\)
The reaction mixture in B consists mainly of products because the equilibrium lies to the right.
Two acidic solutions, X and Y, of equal concentrations have pH values of 2 and 6 respectively.
For each of the reactions A and B, deduce whether water is acting as an acid or a base and explain your answer.
In reaction B, identify the stronger base, \({\text{NH}}_2^ - \) or \({\text{O}}{{\text{H}}^ - }\) and explain your answer.
In reactions A and B, identify the stronger acid, \({\text{NH}}_4^ + \) or \({\text{N}}{{\text{H}}_{\text{3}}}\) (underlined) and explain your answer.
Describe two different experimental methods to distinguish between aqueous solutions of a strong base and a weak base.
Calculate the hydrogen ion concentrations in the two solutions and identify the stronger acid.
Determine the ratio of the hydrogen ion concentrations in the two solutions X and Y.
Markscheme
acid in both reactions;
because it loses a proton/hydrogen ion/\({{\text{H}}^ + }\) / proton/hydrogen ion/\({{\text{H}}^ + }\) donor;
Second mark can be scored if they do not identify it as an acid in both reactions.
\({\text{NH}}_2^ - \);
more readily accepts a proton / equilibrium lies to the right / takes \({{\text{H}}^ + }\) from \({{\text{H}}_{\text{2}}}{\text{O}}\);
If OH– chosen award [0]
\({\text{NH}}_4^ + \);
donates a proton more readily than \({\text{N}}{{\text{H}}_{\text{3}}}\) / equilibrium lies to the left;
If NH3 chosen award [0]
solutions of the same concentration;
pH meter;
strong base has a higher pH / weak base has lower pH;
indicator paper/U.I solution;
strong base has a higher pH/more purple / weak base has lower pH/blue not purple / OWTTE;
measuring conductivity (with conductivity meter);
strong base has a higher conductivity / weak base has lower conductivity;
comparing heat of neutralisation with acid;
strong base releases more heat / weak base releases less heat;
Award [4 max] for two correct methods with expected results.
X;
\({\text{[X]}} = {10^{ - 2}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\) and \({\text{[Y]}} = {10^{ - 6}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\(10\,000/{10^4}:1\);
Ratio should be in form above.
Examiners report
This was the second most popular question. In (a) many candidates scored marks for their understanding of acid-base behaviour in terms of proton transfer and correctly identified \({{\text{H}}_{\text{2}}}{\text{O}}\) as acting as an acid. Identifying and explaining \({\text{NH}}_2^ - \) as the strongest base and \({\text{NH}}_4^ + \) as the strongest acid proved more problematic.
This was the second most popular question. In (a) many candidates scored marks for their understanding of acid-base behaviour in terms of proton transfer and correctly identified \({{\text{H}}_{\text{2}}}{\text{O}}\) as acting as an acid. Identifying and explaining \({\text{NH}}_2^ - \) as the strongest base and \({\text{NH}}_4^ + \) as the strongest acid proved more problematic.
This was the second most popular question. In (a) many candidates scored marks for their understanding of acid-base behaviour in terms of proton transfer and correctly identified \({{\text{H}}_{\text{2}}}{\text{O}}\) as acting as an acid. Identifying and explaining \({\text{NH}}_2^ - \) as the strongest base and \({\text{NH}}_4^ + \) as the strongest acid proved more problematic.
In (b), in spite of the wording in the question (“experimental methods”) many answers mentioned only a property, such as “a strong base has a higher pH than a weak base”, and several who chose an indicator to distinguish them picked one with only two colours, such as phenolphthalein. Most candidates omitted to mention that the solutions should be of the same concentration. Although most could describe one good method (either pH or conductivity), the second method often involved reaction rates or titrations and descriptions of how these were poor.
In (c), although most were able to convert pH values into \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values, fewer were able to compare them as a ratio in the correct form –10,000:1. Some candidates had difficulty identifying the stronger acid.
In (c), although most were able to convert pH values into \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values, fewer were able to compare them as a ratio in the correct form –10,000:1. Some candidates had difficulty identifying the stronger acid.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
Distinguish between a weak acid and a strong acid.
Weak acid:
Strong acid:
Suggest why it is more convenient to express acidity using the pH scale instead of using the concentration of hydrogen ions.
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(ii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iii) Determine the percentage purity of the hydrated ethanedioic acid sample.
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Markscheme
Weak acid: partially dissociated/ionized «in solution/water»
AND
Strong acid: «assumed to be almost» completely/100% dissociated/ionized «in solution/water»
Accept answers relating to pH, conductivity, reactivity if solutions of equal concentrations stated.
«log scale» reduces a wide range of numbers to a small range
OR
simple/easy to use
OR
converts exponential expressions into linear scale/simple numbers
Do not accept “easy for calculations”
i
«n(NaOH) = \(\left( {\frac{{14.0}}{{1000}}} \right)\) dm-3 x 0.100 mol dm-3 =» 1.40 x 10-3 «mol»
ii
«\(\frac{1}{2} \times 1.40 \times {10^{ - 3}} = \) \(7.00 \times {10^{ - 4}}\) «mol»
iii
ALTERNATIVE 1:
«mass of pure hydrated ethanedioic acid in each titration = 7.00 × 10-4 mol × 126.08 g mol-1 =» 0.0883 / 8.83 × 10-2 «g»
mass of sample in each titration = «\(\frac{{25}}{{1000}}\)×5.00g=»0.125«g»
«% purity = \(\frac{{0.0883{\rm{g}}}}{{0.125{\rm{g}}}}\) × 100 =» 70.6 «%»
ALTERNATIVE 2:
«mol of pure hydrated ethanedioic acid in 1 dm3 solution = 7.00 × 10-4 × \(\frac{{1000}}{{25}}\) =» 2.80×10-2 «mol»
«mass of pure hydrated ethanedioic acid in sample = 2.80 × 10-2 mol × 126.08 g mol-1 =» 3.53 «g»
«% purity = \(\frac{{3.53{\rm{g}}}}{{5.00{\rm{g}}}}\) × 100 =» 70.6 «%»
ALTERNATIVE 3:
mol of hydrated ethanedioic acid (assuming sample to be pure) = \(\frac{{5.00{\rm{g}}}}{{126.08{\rm{gmo}}{{\rm{l}}^{ - 1}}}}\) = 0.03966 «mol»
actual amount of hydrated ethanedioic acid = «7.00 × 10-4 × \(\frac{{1000}}{{25}}\) =» 2.80 × 10-2 «mol»
«% purity = \(\frac{{2.80 \times {{10}^{ - 2}}}}{{0.03966}}\) × 100 =» 70.6 «%»
Award suitable part marks for alternative methods.
Award [3] for correct final answer.
Award [2 max] for 50.4 % if anhydrous ethanedioic acid assumed.
electrons delocalized «across the O–C–O system»
OR
resonance occurs
Accept delocalized π-bond(s).
122 «pm» < C–O < 143 «pm»
Accept any answer in the range 123 «pm» to 142 «pm». Accept “bond intermediate between single and double bond” or “bond order 1.5”.
Examiners report
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
Markscheme
(i) from (pale) green/colourless to yellow/orange/brown;
Initial colour must be stated.
Do not accept “clear/transparent” instead of “colourless”.
(ii) chlorine more reactive/more powerful oxidizing agent (than bromine);
Accept opposite statements for bromine.
Accept “chloride ion a weaker reducing agent” / “bromide ion a stronger reducing agent”.
Accept “chlorine more electronegative than bromine”.
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2NaBr(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2NaCl(aq)}}\) /
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Do not accept with equilibrium sign.
solid (in a colourless solution);
Accept “dark brown solution”.
chloric(I) acid (shown as) a molecule/molecular, but hydrochloric acid (shown as being) split into ions / OWTTE;
Accept “chloric(I) acid is partially dissociated and hydrochloric acid is fully dissociated”.
Reference needed to both acids for mark.
\({\text{HOCl(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}/{\text{HOCl(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}\);
Equilibrium sign required for the mark.
Ignore state symbols.
acid displaces the equilibrium to the left (to form chlorine);
chlorine is toxic/poisonous/harmful/lung irritant;
Accept answers that refer to the (c) (ii) equilibrium.
chloric(I) acid has –OH group / hydrogen attached to a very electronegative atom;
Accept polar molecule.
can form hydrogen bonds to water;
hydrogen bonding to water increases its solubility;
(as a weak acid it is) in equilibrium with ions;
 ;
;
Accept lines, dots or crosses to represent electron pairs.
\( \sim\)104°;
Accept values between 102° and 106°.
four electron pairs/regions of high electron density around O atom / electron pairs/regions of high electron density tetrahedrally arranged and two lone/non-bonding electron pairs on O atom;
Accept Lewis structure with two lone pairs on O and two angular bond pairs if given here as equivalent to M2.
lone pair–bonding pair repulsion greater than bonding pair–bonding pair repulsion;
(i) \({\text{(1) Cl}}{{\text{O}}^ - } + \) 2\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) }}{{\text{H}}_2}{\text{O}} + {\text{(1) C}}{{\text{l}}^ - }\);
\({\text{(1) SO}}_4^{2 - } + \) 4\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) S}}{{\text{O}}_2} + \) 2\(\,{{\text{H}}_2}{\text{O}}\);
(ii) Award [2] for all correct, [1] for 2 or 3 correct.

Remember to apply ECF from previous equations.
Penalize incorrect notation (eg, 4 or 4+ rather than +4) once only, so award [1] for a fully correct answer in an incorrect format.
(iii) \({\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\)
correct reactants and products;
balancing and cancelling \({{\text{e}}^ - }\), \({{\text{H}}^ + }\) and \({{\text{H}}_{\text{2}}}{\text{O}}\);
Ignore state symbols.
Do not penalize equilibrium sign.
Examiners report
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
Calcium nitrate contains both covalent and ionic bonds.
Nitrogen also forms oxides, which are atmospheric pollutants.
State the formula of both ions present and the nature of the force between these ions.
Ions:
Nature of force:
State which atoms are covalently bonded.
Outline the source of these oxides.
State one product formed from their reaction with water.
State one environmental problem caused by these atmospheric pollutants.
Markscheme
\({\text{C}}{{\text{a}}^{2 + }}\) and \({\text{NO}}_3^ - \);
electrostatic (attraction);
Do not accept ionic.
nitrogen/N and oxygen/O;
Do not accept nitrate/NO3–.
Accept atoms in nitrate/NO3–.
produced by high temperature combustion;
Accept combustion/jet/car engines / car exhaust/emissions / lightning / action of bacteria/microorganisms.
Do not accept combustion/burning, cars, planes, jets, factories, power plants etc.
nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\) / nitrous acid/nitric(III) acid/\({\text{HN}}{{\text{O}}_{\text{2}}}\);
Accept “forms acidic solutions / acid rain”.
acid deposition/rain / respiratory problems / corrosion problems / decomposition of ozone layer / photochemical smog / acidification/pollution of lakes / damage to plants/ trees;
Accept “acid rain” in either part (ii) or part (iii) but not both.
Do not accept air pollution.
Examiners report
This question was surprisingly very poorly answered. In part (a), it was distressing to see a large number of candidates who could not write the correct charge or formula of nitrate ion. In addition, the terminology appears to have confused a number of candidates and for the nature of force, ionic bonding was often stated which was incorrect, as electrostatic attraction was required. In (a) (ii), again candidates failed to answer the question and nitrate was commonly given which was not accepted. The question specifically asked for the atoms involved.
This question was surprisingly very poorly answered. In part (a), it was distressing to see a large number of candidates who could not write the correct charge or formula of nitrate ion. In addition, the terminology appears to have confused a number of candidates and for the nature of force, ionic bonding was often stated which was incorrect, as electrostatic attraction was required. In (a) (ii), again candidates failed to answer the question and nitrate was commonly given which was not accepted. The question specifically asked for the atoms involved.
In part (b), the Aim 8 component of AS 3.3.2 was assessed and this was very poorly answered overall. Inevitably, owing to some overlap in assessment statements these concepts would be more familiar to those studying the Environmental Chemistry option, but undoubtedly studying other options assists in other areas, such as organic chemistry. In (b) (i), many candidates gave generic answers such as cars or factories which did not score. In (ii), many incorrect answers were given such as nitrogen oxides, hydrogen or ozone. In (iii), acid rain was frequently seen and many referred to depletion of the ozone layer. However it was extremely disappointing that many candidates gave the greenhouse effect or global warming or air pollution as the answer, which of course scored no marks.
In part (b), the Aim 8 component of AS 3.3.2 was assessed and this was very poorly answered overall. Inevitably, owing to some overlap in assessment statements these concepts would be more familiar to those studying the Environmental Chemistry option, but undoubtedly studying other options assists in other areas, such as organic chemistry. In (b) (i), many candidates gave generic answers such as cars or factories which did not score. In (ii), many incorrect answers were given such as nitrogen oxides, hydrogen or ozone. In (iii), acid rain was frequently seen and many referred to depletion of the ozone layer. However it was extremely disappointing that many candidates gave the greenhouse effect or global warming or air pollution as the answer, which of course scored no marks.
In part (b), the Aim 8 component of AS 3.3.2 was assessed and this was very poorly answered overall. Inevitably, owing to some overlap in assessment statements these concepts would be more familiar to those studying the Environmental Chemistry option, but undoubtedly studying other options assists in other areas, such as organic chemistry. In (b) (i), many candidates gave generic answers such as cars or factories which did not score. In (ii), many incorrect answers were given such as nitrogen oxides, hydrogen or ozone. In (iii), acid rain was frequently seen and many referred to depletion of the ozone layer. However it was extremely disappointing that many candidates gave the greenhouse effect or global warming or air pollution as the answer, which of course scored no marks.
A student decided to determine the molecular mass of a solid monoprotic acid, HA, by titrating a solution of a known mass of the acid.
The following recordings were made.
Calculate the mass of the acid and determine its absolute and percentage uncertainty.
This known mass of acid, HA, was then dissolved in distilled water to form a \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) solution in a volumetric flask. A \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) sample of this solution reacted with \({\text{12.1 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH solution. Calculate the molar mass of the acid.
The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen. Determine its empirical formula.
A solution of HA is a weak acid. Distinguish between a weak acid and a strong acid.
Describe an experiment, other than measuring the pH, to distinguish HA from a strong acid of the same concentration and describe what would be observed.
Markscheme
0.675 (g) ± 0.002 (g);
Percentage uncertainty: 0.3%;
Accept answers correct to one, two or three significant figures for percentage uncertainty.
In 25.0 cm3: \({n_{{\text{HA}}}} = 1.21 \times {10^{ - 3}}{\text{ (mol)}}\);
In 100 cm3: \({n_{{\text{HA}}}} = 4.84 \times {10^{ - 3}}{\text{ (mol)}}\);
\({\text{M }}\left( { = \frac{{0.675}}{{4.84 \times {{10}^{ - 3}}}}} \right) = 139{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Accept suitable alternative methods.
\({n_{\text{C}}}:{\text{ }}\left( {\frac{{70.56}}{{12.01}} = } \right){\text{ }}5.88\) and \({n_{\text{O}}}:{\text{ }}\left( {\frac{{23.50}}{{16}} = } \right){\text{ }}1.47\) and \({n_{\text{H}}}:{\text{ }}\left( {\frac{{5.94}}{{1.01}} = } \right){\text{ }}5.88\)
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{4}}}{\text{O}}\);
Award [2] for correct final answer.
Accept answers using integer values of molar mass.
weak acids partially dissociated/ionized and strong acids completely dissociated/ionized (in solution/water) / OWTTE;
strong acids have greater electrical conductivity / weak acids have lower electrical conductivity;
OR
adding a reactive metal / carbonate / hydrogen carbonate;
Accept correct example.
stronger effervescence with strong acids / weaker with weak acids / OWTTE;
OR
adding a strong base;
Accept correct example.
strong acid would increase more in temperature / weak acids increase less in temperature;
Examiners report
Many students lost easy marks as they forgot to propagate uncertainties.
Many candidates struggled with the concept of mole and the dilution factor added to the difficulty.
Most students determined the empirical formula correctly.
Weak and strong acids were generally correctly defined, though sometimes they were defined in terms of pH.
The conductivity test appeared frequently and was well described. Many candidates used a strong based, but then went on to describe a titration method.
When nitrogen gas and hydrogen gas are allowed to react in a closed container, the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\;\;\;\;\;\Delta H = - 92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst. Explain the effect of a catalyst on the rate of reaction.
Sketch the Maxwell–Boltzmann energy distribution curve for a reaction, labelling both axes and showing the activation energy with and without a catalyst.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted-Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
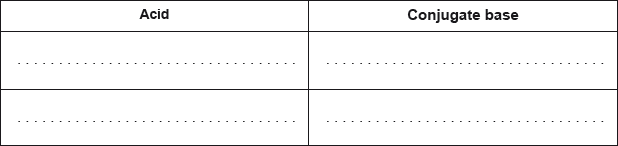
Outline an experiment and its results which could be used to distinguish between a strong base and a weak base.
Markscheme
rates of forward and reverse reactions are equal / opposing changes occur at equal rates;
the concentrations of all reactants and products remain constant / macroscopic properties remain constant;
closed/isolated system;
Accept “the same” for “equal” in M1 and for “constant” in M2.
\(({K_{\text{c}}} = )\frac{{{{{\text{[N}}{{\text{H}}_3}{\text{(g)]}}}^2}}}{{{\text{[}}{{\text{N}}_2}{\text{(g)]}} \times {{{\text{[}}{{\text{H}}_2}{\text{(g)]}}}^3}}}\);
Ignore state symbols.
Concentration must be represented by square brackets.
The volume of the container is increased:
position of equilibrium shifts to the left/reactants and fewer moles of gas on the right hand side/pressure decreases / OWTTE;
Ammonia is removed from the equilibrium mixture:
position of equilibrium shifts to the right/products and [NH3] decreases so [N2] and [H2] must also decrease to keep Kc constant
OR
position of equilibrium shifts to the right/products and rate of reverse reaction decreases / OWTTE;
Award [1 max] if both predicted changes are correct.
Do not accept “to increase [NH3]” or reference to LCP without explanation.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction;
Accept “energy difference between reactants and transition state”.
rate increases;
more effective/successful collisions per unit time / greater proportion of collisions effective;
alternative pathway and a lower activation energy
OR
lowers activation energy so that more particles have enough energy to react;
Do not accept just “lowers/reduces the activation energy”.
Accept “provides a surface for reacting/reactants/reaction”.
Curve showing:
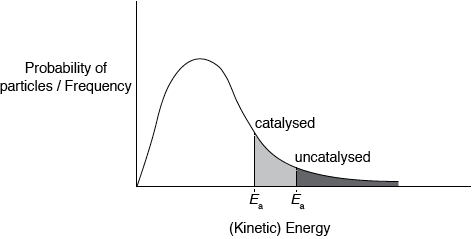
general shape of Maxwell-Boltzmann energy distribution curve and labelled y-axis: probability of particles / frequency and labelled x-axis: (kinetic)energy;
Curve must begin at zero and must not cut the x-axis on the RHS.
Accept number/fraction/proportion of particles for y-axis label, but do not accept amount or just particles.
correct position of \({E_{\text{a}}}\) catalysed and \({E_{\text{a}}}\) uncatalysed;
Shading shown in the diagram is not required for the marks.
(i) slower rate / OWTTE;
uneconomic / OWTTE;
(ii) high cost for building/maintaining plant / high energy cost of compressor /OWTTE;
Do not accept “high pressure is expensive” without justification.
Accept high pressure requires high energy.
electron pair donor;
Accept lone pair donor.
proton acceptor and partially/slightly ionized;
Accept “proton acceptor and partially/slightly dissociated”.
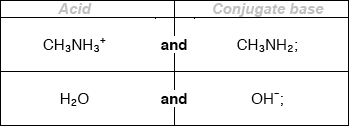
Award [1 max] for two correct acids OR two correct conjugate bases.
solutions of equal concentration;
pH measurement/UIP;
strong base has higher pH;
OR
solutions of equal concentration;
electrical conductivity measurement;
strong base has higher electrical conductivity;
OR
solutions of equal concentration;
temperature difference in neutralization reaction with a strong acid;
strong base has a greater temperature difference;
Accept reverse arguments for observations.
Examiners report
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
This was, by far and away, the most common choice for Section B.
The conditions for an equilibrium system were well known, and the \({K_{\text{c}}}\) expression was almost universally correctly given, the incidence of curved brackets was very low. With the description of the effect of changing conditions, the increase in volume change generally scored, but the answers for the removal of ammonia were far too general to be given credit. It is pleasing to note that most candidates are aware of the importance of using the word “'minimum”, as well as the effect of a catalyst, with most giving perfect answers. The drawing of the Maxwell-Boltzmann energy distribution curve suffered from poor draughtsmanship. Too many curves did not start at the origin and lacked correct labels. An appreciable minority drew the energy/reaction co-ordinate graph. The knowledge of the compromise conditions for the Haber process was often confused, particularly with regard to why high pressure is not used, where far too many answers lacked the depth required. Occasionally the word “pair” was missing for the definition of a Lewis base, and with the definition of a weak Brønsted-Lowry base most candidates failed to appreciate the difference between partially/slightly ionized and “not completely” ionized, the part of proton acceptor was also often missed out. With the description of the experiment to show the difference between a strong and weak base, many scored two out of the three available; the concept of a fair test, and the importance of equal concentrations was rarely appreciated.
Across period 3, elements increase in atomic number, decrease in atomic radius and increase in electronegativity.
Define the term electronegativity.
Explain why the atomic radius of elements decreases across the period.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Suggest the pH of the solutions formed in part (c) (i).
Describe three tests that can be carried out in the laboratory, and the expected results, to distinguish between \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) and \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\).
Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
Describe the bonding and structure of sodium chloride.
State the formula of the compounds formed between the elements below.
Sodium and sulfur:
Magnesium and phosphorus:
Covalent bonds form when phosphorus reacts with chlorine to form \({\text{PC}}{{\text{l}}_{\text{3}}}\). Deduce the Lewis (electron dot) structure, the shape and bond angle in \({\text{PC}}{{\text{l}}_{\text{3}}}\) and explain why the molecule is polar.
Lewis (electron dot) structure:
Name of shape:
Bond angle:
Explanation of polarity of molecule:
Markscheme
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons;
Do not accept “element” instead of “atom/nucleus”.
Do not accept “electrons” alone.
increasing nuclear charge/increasing number of protons / increased attraction of (valence) electrons to nucleus;
electrons added are in same (outer) energy level;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
Accept \(N{a_2}O(s) + {H_2}O(l) \to 2N{a^ + }(aq) + 2O{H^ - }(aq)\).
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_3}{\text{(aq)}}\);
Accept \({P_2}{O_5}(s) + 3{H_2}O(l) \to 2{H_3}P{O_4}(aq)\).
Accept \({P_4}{O_{10}}(s) + 6{H_2}O(l) \to 4{H^ + }(aq) + 4{H_2}PO_4^ - (aq)\).
Ignore state symbols.
NaOH: > 7;
Accept any pH greater than 7.
H3PO4: < 7;
Accept any pH less than 7.
Award [1 max] if stated that “NaOH alkali/basic and H3PO4 acidic”, but pH values not given.
measuring electrical conductivity and strong acids have greater electrical
conductivity/weak acids have lower electrical conductivity;
Do not accept conductivity for electrical conductivity.
Accept explanation in terms of lightbulb in circuit.
measure pH/use universal indicator and pH higher for weak acid/pH lower for strong acid;
conduct titration with a strong base and equivalence point higher for weak acid / buffer region for weak acid;
adding a reactive metal/carbonate/hydrogen carbonate and stronger effervescence/faster reaction with strong acids;
Accept converse argument.
Accept correct example.
adding a strong base and strong acid would increase more in temperature/weak acids increase less in temperature;
Accept correct example.
Award [1 max] for three suitable tests without correct results.
Accept specific examples with given strong acid and weak acid.
Accept “addition of AgNO3 (aq) and white precipitate with HCl (aq)”.
Do not accept “smell”.
Lewis acid (only);
electron pair acceptor / not a proton donor;
Bonding: (electrostatic) attraction between oppositely charged ions;
Do not accept ionic bonding without some description.
Structure: lattice/giant structure of ions / each \({\text{N}}{{\text{a}}^ + }\) surrounded by \({\text{6 C}}{{\text{l}}^ - }\) (and vice-versa);
\({\text{N}}{{\text{a}}_2}{\text{S}}\);
\({\text{M}}{{\text{g}}_3}{{\text{P}}_2}\);
Lewis structure:
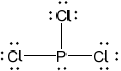 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
Do not award the mark if lone pairs are missing.
Name of shape:
(trigonal/triangular) pyramidal;
Bond angle:
\( < 109.5^\circ \);
Accept any value within the range 100°−109°.
Literature value is 100°.
Explanation of polarity:
dipoles do not cancel (as molecule is not symmetrical) / there is a net dipole (as molecule is not symmetrical) / unsymmetrical distribution of charge;
Accept suitable labelled diagram.
No ECF if original structure is incorrect.
Examiners report
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
A group of students investigated the rate of the reaction between aqueous sodium thiosulfate and hydrochloric acid according to the equation below.
\[{\text{N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(g)}} + {\text{S(s)}} + {{\text{H}}_2}{\text{O(l)}}\]
The two reagents were rapidly mixed together in a beaker and placed over a mark on a piece of paper. The time taken for the precipitate of sulfur to obscure the mark when viewed through the reaction mixture was recorded.
Initially they measured out \({\text{10.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid and then added \({\text{40.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.0200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium thiosulfate. The mark on the paper was obscured 47 seconds after the solutions were mixed.
The teacher asked the students to measure the effect of halving the concentration of sodium thiosulfate on the rate of reaction.
The teacher asked the students to devise another technique to measure the rate of this reaction.
Another group suggested collecting the sulfur dioxide and drawing a graph of the volume of gas against time.
The teacher made up \({\text{2.50 d}}{{\text{m}}^{\text{3}}}\) of the sodium thiosulfate solution using sodium thiosulfate pentahydrate crystals, \({\text{N}}{{\text{a}}_2}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}}\). Calculate the required mass of these crystals.
(i) State the volumes of the liquids that should be mixed.
(ii) State why it is important that the students use a similar beaker for both reactions.
(iii) Explain, in terms of the collision theory, how decreasing the concentration of sodium thiosulfate would affect the time taken for the mark to be obscured.
(i) Sketch and label, indicating an approximate activation energy, the Maxwell–Boltzmann energy distribution curves for two temperatures, \({T_1}\) and \({T_2}{\text{ }}({T_2} > {T_1})\), at which the rate of reaction would be significantly different.

(ii) Explain why increasing the temperature of the reaction mixture would significantly increase the rate of the reaction.
(i) One group suggested recording how long it takes for the pH of the solution to change by one unit. Calculate the initial pH of the original reaction mixture.
(ii) Deduce the percentage of hydrochloric acid that would have to be used up for the pH to change by one unit.
(i) Calculate the volume of sulfur dioxide, in \({\text{c}}{{\text{m}}^{\text{3}}}\), that the original reaction mixture would produce if it were collected at \(1.00 \times {10^5}{\text{ Pa}}\) and 300 K.
(ii) Suggest why it is better to use a gas syringe rather than collecting the gas in a measuring cylinder over water.
Markscheme
\({\text{mol N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}( = 2.50 \times 0.0200) = 0.0500\);
\({{\text{M}}_{\text{r}}}{\text{N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3} \bullet {\text{5}}{{\text{H}}_2}{\text{O}}\left( { = (2 \times 22.99) + (2 \times 32.06) + (3 \times 16.00) + (5 \times 18.02)} \right) = 248.20\);
Allow 248.
\({\text{mass N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3} \bullet {\text{5}}{{\text{H}}_2}{\text{O}} = (0.0500 \times 248.20) = 12.4{\text{ g}}\);
Award [3] for correct final answer.
Award [2] for 7.91g (water of crystallization omitted in \({M_r}\) calculation).
(i) 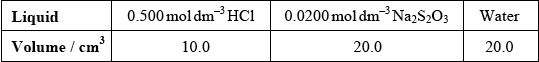 ;
;
Accept other volumes in a 1:2:2 ratio.
(ii) depth of liquid in the beaker must remain constant / OWTTE;
Accept “same thickness of glass” and any other valid point, such as answers framed around minimizing uncontrolled variables / making it a “fair test”.
(iii) increases the time;
decrease in collision frequency/number of collisions per unit time;
Do not award mark for decrease in number of collisions.
(i) 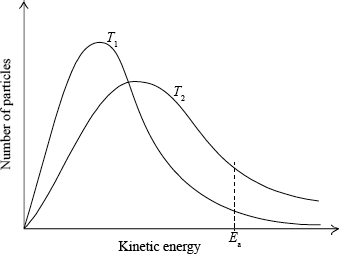
labelled y-axis: number of particles / probability of particles (with that kinetic energy) and labelled x-axis: (kinetic) energy;
Allow fraction/proportion/amount of particles (with kinetic energy) for y-axis label.
Allow speed/velocity for x-axis label.
T2 curve broader and with maximum lower and to right of T1 curve;
Do not award this mark if both curves not asymmetric.
Curves must pass through the origin and be asymptotic to x axis.
Do not award this mark if curves not labelled.
\({E_{\text{a}}}\) marked on graph;
(ii) kinetic energy of molecules increases;
This may be answered implicitly in the final marking point.
frequency of collision/number of collisions per unit time increases;
Only penalize use of “number of collisions” if not penalized in (b)(iii).
greater proportion of molecules have energy greater than/equal to activation energy / rate related to temperature by the Arrhenius equation;
Award [1 max] for statements such as “there will be more successful collisions” if neither of last two marking points awarded.
(i) \({\text{[}}{{\text{H}}^ + }{\text{]}} = 0.5 \times \frac{{10}}{{{\text{50}}}} = 0.1{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\({\text{pH }}\left( { = - \log {\text{[}}{{\text{H}}^ + }{\text{]}} = - \log (0.10)} \right) = 1\);
(ii) 90%;
(i) \({\text{mol N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3} = {\text{mol S}}{{\text{O}}_2} = 0.0400 \times 0.0200 = 0.000800\);
\(V = \frac{{n \times R \times T}}{P}/\frac{{0.000800 \times 8.31 \times 300}}{{{{10}^5}}}\);
\((1.99 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}) = 19.9{\text{ }}({\text{c}}{{\text{m}}^3})\);
Award [3] for correct final answer.
Accept 20.0 cm3 if R = 8.314 is used.
Award [2] for 17.9 cm3 or 19.2 cm3 (result from using molar volume at standard temperature and pressure or at room temperature and pressure).
OR
\({\text{mol N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3} = {\text{mol S}}{{\text{O}}_2} = 0.0400 \times 0.0200 = 0.000800\);
\(V = 0.00080 \times 2.24 \times {10^{ - 2}} \times \left[ {\frac{{1.00 \times {{10}^5}}}{{1.01 \times {{10}^5}}}} \right] \times \frac{{300}}{{273}}\);
\((1.95 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}) = 19.5{\text{ }}({\text{c}}{{\text{m}}^3})\);
Award [3] for correct final answer.
Deduct [1] for answers based on amount of HCl, so correct calculation would score [2 max].
(ii) sulfur dioxide is soluble in water;
Accept other reasonable responses based on sound chemistry.
Accept “syringe more accurate/precise” or “less gas escapes”.
Examiners report
This was quite a popular question, but responses were mixed. As in question 1, students struggled to answer questions with a strong practical context, with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and responses for the need for similar beakers to be used were often too vague. Explanations of changes of rates in terms of the collision theory were generally successful but a significant number referred to the “number” rather than “frequency” of collisions. Many candidates were able to sketch Maxwell–Boltzmann distribution curves for the two temperatures, \({T_1}\) and \({T_2}\), but marks were lost due to careless omissions; the graphs did not start at the origin, were not labelled or the activation energy was missing. Many struggled to calculate the pH and many teachers have commented that this question was beyond what is expected at Standard Level and it is acknowledged that the question would have been more accessible if candidates had been asked to calculate the concentration of \({{\text{H}}^ + }\) ions and state the pH. In part (e) many students could quote and substitute into the ideal gas equation, correctly converting the temperature to Kelvin, but converting from \({{\text{m}}^{\text{3}}}\) to \({\text{c}}{{\text{m}}^{\text{3}}}\) posed a problem for most candidates. Although not necessary for the mark, as answers which referred to improved accuracy and precision were accepted, most candidates did not refer to the solubility of sulfur dioxide as a problem when using measuring cylinders to measure its volume.
This was quite a popular question, but responses were mixed. As in question 1, students struggled to answer questions with a strong practical context, with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and responses for the need for similar beakers to be used were often too vague. Explanations of changes of rates in terms of the collision theory were generally successful but a significant number referred to the “number” rather than “frequency” of collisions. Many candidates were able to sketch Maxwell–Boltzmann distribution curves for the two temperatures, \({T_1}\) and \({T_2}\), but marks were lost due to careless omissions; the graphs did not start at the origin, were not labelled or the activation energy was missing. Many struggled to calculate the pH and many teachers have commented that this question was beyond what is expected at Standard Level and it is acknowledged that the question would have been more accessible if candidates had been asked to calculate the concentration of \({{\text{H}}^ + }\) ions and state the pH. In part (e) many students could quote and substitute into the ideal gas equation, correctly converting the temperature to Kelvin, but converting from \({{\text{m}}^{\text{3}}}\) to \({\text{c}}{{\text{m}}^{\text{3}}}\) posed a problem for most candidates. Although not necessary for the mark, as answers which referred to improved accuracy and precision were accepted, most candidates did not refer to the solubility of sulfur dioxide as a problem when using measuring cylinders to measure its volume.
This was quite a popular question, but responses were mixed. As in question 1, students struggled to answer questions with a strong practical context, with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and responses for the need for similar beakers to be used were often too vague. Explanations of changes of rates in terms of the collision theory were generally successful but a significant number referred to the “number” rather than “frequency” of collisions. Many candidates were able to sketch Maxwell–Boltzmann distribution curves for the two temperatures, \({T_1}\) and \({T_2}\), but marks were lost due to careless omissions; the graphs did not start at the origin, were not labelled or the activation energy was missing. Many struggled to calculate the pH and many teachers have commented that this question was beyond what is expected at Standard Level and it is acknowledged that the question would have been more accessible if candidates had been asked to calculate the concentration of \({{\text{H}}^ + }\) ions and state the pH. In part (e) many students could quote and substitute into the ideal gas equation, correctly converting the temperature to Kelvin, but converting from \({{\text{m}}^{\text{3}}}\) to \({\text{c}}{{\text{m}}^{\text{3}}}\) posed a problem for most candidates. Although not necessary for the mark, as answers which referred to improved accuracy and precision were accepted, most candidates did not refer to the solubility of sulfur dioxide as a problem when using measuring cylinders to measure its volume.
This was quite a popular question, but responses were mixed. As in question 1, students struggled to answer questions with a strong practical context, with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and responses for the need for similar beakers to be used were often too vague. Explanations of changes of rates in terms of the collision theory were generally successful but a significant number referred to the “number” rather than “frequency” of collisions. Many candidates were able to sketch Maxwell–Boltzmann distribution curves for the two temperatures, \({T_1}\) and \({T_2}\), but marks were lost due to careless omissions; the graphs did not start at the origin, were not labelled or the activation energy was missing. Many struggled to calculate the pH and many teachers have commented that this question was beyond what is expected at Standard Level and it is acknowledged that the question would have been more accessible if candidates had been asked to calculate the concentration of \({{\text{H}}^ + }\) ions and state the pH. In part (e) many students could quote and substitute into the ideal gas equation, correctly converting the temperature to Kelvin, but converting from \({{\text{m}}^{\text{3}}}\) to \({\text{c}}{{\text{m}}^{\text{3}}}\) posed a problem for most candidates. Although not necessary for the mark, as answers which referred to improved accuracy and precision were accepted, most candidates did not refer to the solubility of sulfur dioxide as a problem when using measuring cylinders to measure its volume.
This was quite a popular question, but responses were mixed. As in question 1, students struggled to answer questions with a strong practical context, with very few able to devise a mixture that would halve the concentration of thiosulfate, whilst keeping other concentrations constant, and responses for the need for similar beakers to be used were often too vague. Explanations of changes of rates in terms of the collision theory were generally successful but a significant number referred to the “number” rather than “frequency” of collisions. Many candidates were able to sketch Maxwell–Boltzmann distribution curves for the two temperatures, \({T_1}\) and \({T_2}\), but marks were lost due to careless omissions; the graphs did not start at the origin, were not labelled or the activation energy was missing. Many struggled to calculate the pH and many teachers have commented that this question was beyond what is expected at Standard Level and it is acknowledged that the question would have been more accessible if candidates had been asked to calculate the concentration of \({{\text{H}}^ + }\) ions and state the pH. In part (e) many students could quote and substitute into the ideal gas equation, correctly converting the temperature to Kelvin, but converting from \({{\text{m}}^{\text{3}}}\) to \({\text{c}}{{\text{m}}^{\text{3}}}\) posed a problem for most candidates. Although not necessary for the mark, as answers which referred to improved accuracy and precision were accepted, most candidates did not refer to the solubility of sulfur dioxide as a problem when using measuring cylinders to measure its volume.
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid were added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
(i) Identify the reagent necessary for this reaction to occur.
(ii) Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
Determine the enthalpy change, in kJ mol\(^{ - 1}\), for this reaction, using Table 10 of the Data Booklet.
Bromoethene, \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHBr}}\), can undergo polymerization. Draw a section of this polymer that contains six carbon atoms.
Markscheme
HCl is a strong acid and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) is a weak acid so HCl has higher conductivity / HCl dissociates completely in water and \({\text{C}}{{\text{H}}_3}{\text{COOH}}\) does not, so HCl has higher conductivity / HCl is stronger acid (than \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) so has higher \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and higher conductivity;
(i) \({\text{C}}{{\text{H}}_3}{\text{COOH(aq)}} + {\text{HCO}}_3^ - {\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept NaHCO3(aq) and CH3COONa (aq) instead of ions.
Ignore state symbols.
(ii) \(n{\text{(C}}{{\text{H}}_3}{\text{COOH)}} = 0.00500{\text{ (mol)}}\) and \(n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\({\text{NaHC}}{{\text{O}}_3}\) is limiting;
(iii) \(n{\text{(C}}{{\text{O}}_2}{\text{)}} = n{\text{(NaHC}}{{\text{O}}_3}{\text{)}} = 0.00450{\text{ (mol)}}\);
\(m{\text{(C}}{{\text{O}}_2}{\text{)}} = 0.00450 \times 44.01 = 0.198{\text{ (g)}}\);
Award [2] for correct final answer.
(i) \(T = 363{\text{ K}}\) and \(V = 9.50 \times {10^{ - 5}}{\text{ }}{{\text{m}}^3}\);
Accept V = 9.5 \( \times \) 10–2 dm3 if P is used as 101 kPa in calculation.
\(n = \frac{{PV}}{{RT}} = \frac{{1.01 \times {{10}^5} \times 9.50 \times {{10}^{ - 5}}}}{{8.31 \times 363}}\);
\( = 3.18 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [3] for correct final answer.
(ii) \(M = \left( {\frac{m}{n} = \frac{{0.348}}{{3.18 \times {{10}^{ - 3}}}} = } \right)109{\text{ }}({\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
(i) (dilute aqueous) NaOH/sodium hydroxide / KOH/potassium hydroxide;
Do not accept hydroxide/OH–.
(ii) 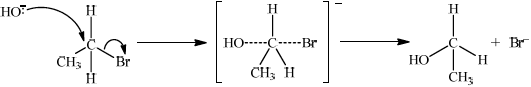
curly arrow going from lone pair/negative charge on O in HO– to C;
Do not allow curly arrow originating on H in HO–.
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH—C bond is represented.
bonds broken:
1(C=C) \( + 1\) (H–Br) / \((612 + 366 = )978{\text{ (kJ)}}\);
Accept 2630 (kJ).
bonds formed:
1(C–C) \( + 1\) (C–H) \( + 1\) (C–Br) / \((1 \times 347 + 1 \times 413 + 1 \times 290 = )1050{\text{ (kJ)}}\);
Accept 2702 (kJ).
\(\Delta H = - 72{\text{ }}({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Award [3] for correct final answer.
Award [2 max] for +72 (kJ mol−1).
 ;
;
Extension bonds required.
Ignore brackets and n.
Examiners report
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Question 7 was answered by relatively few candidates, and those who chose this question were usually not well-prepared. In (a) very few candidates indicated that HCl is a strong acid and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\) a weak one. Many candidates seemed unfamiliar with the distinction between state and outline and simply said that HCl would be a better conductor. In (b)(i) very few candidates could state a correct equation for the reaction between ethanoic acid and sodium hydrogencarbonate, even when the formulas were provided, but most could calculate the limiting reagent in (b)(ii) and the mass of \({\text{C}}{{\text{O}}_{\text{2}}}\) produced in (b)(iii). Part (c) gave details of a volatile organic liquid. Most candidates could calculate the moles of gas present in (c)(i), although the conversion to the correct units for pressure and volume gave many problems. The calculation of the molar mass of the gas, especially with ECF applied, was generally done well by the candidates. Part (d) referred to the substitution reaction of bromoethane to form ethanol. Identifying the reagent in (d)(i) for this reaction caused problems, with many stating \({\text{O}}{{\text{H}}^ - }\) as the reagent instead of NaOH or KOH. Only the best candidates could draw the mechanism for this substitution reaction in (d)(ii). Many candidates seemed to have very little idea of how to represent an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Although most candidates identified HBr as the reagent which could produce bromoethane from ethene, they often gave UV as the required condition in (e)(i). Teachers should note that assessment statement 10.6.1 indicates that reagents, conditions and equations should be included for all reaction types listed in the syllabus. Calculation of the enthalpy change using bond enthalpies did not give problems to the good candidates in (e)(ii) but many of the weaker candidates failed to identify all the bonds broken and formed, and only scored the final mark through the application of ECF. Drawing a section of a polymer produced from bromoethene in (e)(iii) presented few problems for most candidates.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a weak base.
Iron is more reactive than copper.
Draw the Lewis structure of ammonia and state the shape of the molecule and its bond angles.
The conjugate acid of ammonia is the ammonium ion, \({\text{NH}}_4^ + \). Draw the Lewis structure of the ammonium ion and deduce its shape and bond angles.
Describe two different properties that could be used to distinguish between a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a strong monoprotic acid and a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a weak monoprotic acid.
Explain, using the Brønsted-Lowry theory, how water can act either as an acid or a base. In each case identify the conjugate acid or base formed.
Draw a labelled diagram of a voltaic cell made from an \({\text{Fe(s)}}/{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) half-cell connected to a \({\text{Cu(s)}}/{\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) half-cell. In your diagram identify the positive electrode (cathode), the negative electrode (anode) and the direction of electron flow in the external circuit.
Deduce the half-equations for the reactions taking place at the positive electrode (cathode) and negative electrode (anode) of this voltaic cell.
Deduce the overall equation for the reaction taking place in the voltaic cell and determine which species acts as the oxidizing agent and which species has been reduced.
Markscheme
 ;
;
Accept any combination of dots/crosses and lines to represent electron pairs.
(trigonal/triangular) pyramid;
Allow 3D representation using wedges and dotted bonds of trigonal pyramidal molecule.
107°;
Accept any angle between 105° and 108.5°.
No ECF for shape based on incorrect Lewis structure.
 ;
;
Charge needed for mark.
Allow a 3D representation using wedges and dotted bonds of tetrahedral molecule.
109.5°/109°/109° 28';
No ECF for shape based on incorrect Lewis structure.
(measuring) the pH / the strong acid solution will have a lower pH;
conductivity (measurement) / the strong acid will be a better conductor;
the strong acid will react more vigorously with metals/carbonates / the reaction with metals/carbonates;
the heat change when it is neutralized with a base will be different / heat of neutralization / OWTTE;
water can act as a Brønsted-Lowry acid by donating a proton/\({{\text{H}}^ + }\) to form \({\text{O}}{{\text{H}}^ - }\);
water can act as a Brønsted-Lowry base by accepting a proton/\({{\text{H}}^ + }\) to form \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
Accept equations showing the above clearly labelling the acid and basic behaviour and the conjugate acid or base.
Award [1 max] for correct definition of how water can act as a Brønsted-Lowry acid or base.
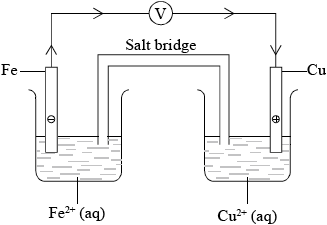
correct diagram including voltmeter/meter, 4 correct species (state symbols not required) and connecting wires;
No credit if wires to electrodes immersed in the solutions.
labelled salt bridge;
Do not accept name of salt (e.g. potassium nitrate) in place of salt bridge.
correctly labelled electrodes (+)/cathode and (–)/anode;
flow of electrons from Fe to Cu in external circuit;
positive electrode: \({\text{C}}{{\text{u}}^{2 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{Cu}}\);
negative electrode: \({\text{Fe}} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{2}}{{\text{e}}^ - }\);
Award [1] if equations correct but at wrong electrodes or if electrodes are missing.
Award [2] for correct equations if electrodes are missing but were correctly labelled in diagram.
Accept e instead of \({e^ - }\).
Ignore state symbols.
Penalize \( \rightleftharpoons \) once only in equations in (ii) and (iii).
\({\text{Fe}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{Cu}}\);
Ignore state symbols.
\({\text{C}}{{\text{u}}^{2 + }}\) is the oxidizing agent and the species that is reduced;
Examiners report
Candidates could draw the Lewis structures in part (a) and generally they could name the shape and suggest the bond angle.
Most knew what a Lewis acid was but some were careless in their definition and said it was an electron acceptor instead of an electron pair acceptor.
Generally candidates could suggest ways of distinguishing between strong and weak acids using pH or conductivity.
The final part of this question caused some difficulty though as students found it hard to show water acting as an acid and a base even though many could correctly state that an acid is a proton donor and a base is a proton acceptor.
Part (b) focused on electrochemistry and although some candidates were able to score 4 marks most lost marks for their diagrams which were often incomplete and/or incorrectly annotated.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly. This carried through to the final part of the question and those that could write the half equations could generally write the overall equation. Identifying the oxidizing agent and the species that has been reduced proved tricky as students were reluctant to suggest the same species- \({\text{C}}{{\text{u}}^{2 + }}\), also some students just said copper which was not specific enough to gain the mark.
Limescale, CaCO3(s), can be removed from water kettles by using vinegar, a dilute solution of ethanoic acid, CH3COOH(aq).
Predict, giving a reason, a difference between the reactions of the same concentrations of hydrochloric acid and ethanoic acid with samples of calcium carbonate.
Dissolved carbon dioxide causes unpolluted rain to have a pH of approximately 5, but other dissolved gases can result in a much lower pH. State one environmental effect of acid rain.
Markscheme
slower rate with ethanoic acid
OR
smaller temperature rise with ethanoic acid
[H+] lower
OR
ethanoic acid is partially dissociated
OR
ethanoic acid is weak
Accept experimental observations such as “slower bubbling” or “feels less warm”.
[2 marks]
Any one of:
corrosion of materials/metals/carbonate materials
destruction of plant/aquatic life
«indirect» effect on human health
Accept “lowering pH of oceans/lakes/waterways”.
[1 mark]
Examiners report
Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and water.
(i) 200.0 g of air was heated by the energy from the complete combustion of 1.00 mol phosphine. Calculate the temperature rise using section 1 of the data booklet and the data below.
Standard enthalpy of combustion of phosphine,
Specific heat capacity of air = 1.00Jg−1K−1 = 1.00 kJkg−1K−1
(ii) The oxide formed in the reaction with air contains 43.6 % phosphorus by mass. Determine the empirical formula of the oxide, showing your method.
(iii) The molar mass of the oxide is approximately 285gmol−1. Determine the molecular formula of the oxide.
(i) State the equation for the reaction of this oxide of phosphorus with water.
(ii) Predict how dissolving an oxide of phosphorus would affect the pH and electrical conductivity of water.
pH:
Electrical conductivity:
(iii) Suggest why oxides of phosphorus are not major contributors to acid deposition.
(iv) The levels of sulfur dioxide, a major contributor to acid deposition, can be minimized by either pre-combustion and post-combustion methods. Outline one technique of each method.
Pre-combustion:
Post-combustion:
Markscheme
(i)
temperature rise «\(\frac{{750 \times 1.00}}{{0.2000 \times 1.00}}\)= 3750 «°C/K»
Do not accept −3750.
(ii)
n(P)«=\(\frac{{43.6}}{{30.97}}\)»=1.41«mol»
n(O)«=\(\frac{{100 - 43.6}}{{16.00}}\)»= 3.53«mol»
\(\frac{{n\left( {\rm{O}} \right)}}{{n\left( {\rm{P}} \right)}} = \frac{{3.53}}{{1.41}} = 2.50\) so empirical formula is» P2O5
Accept other methods where the working is shown.
(iii)
\(\frac{{285}}{{141.9}}\)=2.00, so molecular formula=2×P2O5=»P4O10
(i)
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept P4O10 (s) + 2H2O (l) → 4HPO3 (aq) (initial reaction)
Accept P2O5 (s) + 3H2O (l) → 2H3PO4 (aq)
Accept equations for P4O6 /P2O3 if given in a (iii).
Accept any ionized form of the acids as the products.
pH: decreases AND electrical conductivity: increases.
(iii)
phosphorus not commonly found in fuels
OR
no common pathways for phosphorus oxides to enter the air
OR
amount of phosphorus-containing organic matter undergoing anaerobic decomposition is small
Accept “phosphorus oxides are solids so are not easily distributed in the atmosphere”.
Accept “low levels of phosphorus oxide in the air”. Do not accept “H3PO4 is a weak acid”.
(iv)
Pre-combustion:
remove sulfur/S/sulfur containing compounds
Post-combustion:
remove it/SO2 by neutralization/reaction with alkali/base
Accept “lime injection fluidised bed combustion” for either, but not both.
Examiners report
Graphing is an important tool in the study of rates of chemical reactions.
Excess hydrochloric acid is added to lumps of calcium carbonate. The graph shows the volume of carbon dioxide gas produced over time.
Sketch a Maxwell–Boltzmann distribution curve for a chemical reaction showing the activation energies with and without a catalyst.
Sketch a curve on the graph to show the volume of gas produced over time if the same mass of crushed calcium carbonate is used instead of lumps. All other conditions remain constant.
State and explain the effect on the rate of reaction if ethanoic acid of the same concentration is used in place of hydrochloric acid.
Outline why pH is more widely used than [H+] for measuring relative acidity.
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
Markscheme
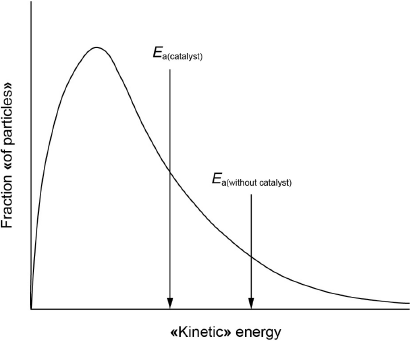
both axes correctly labelled
correct shape of curve starting at origin
Ea(catalyst) < Ea(without catalyst) on x-axis
M1:
Accept “speed” for x-axis label.
Accept “number of particles”, “N”, “frequency” or “probability «density»” for y-axis label.
Do not accept “potential energy” for x-axis label.
M2:
Do not accept a curve that touches the x-axis at high energy.
Do not award M2 if two curves are drawn.
M3:
Ignore any shading under the curve.
[3 marks]
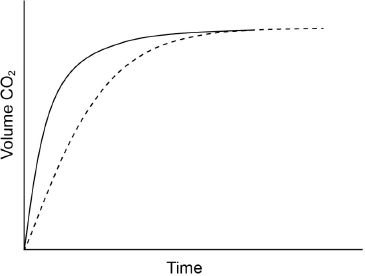
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
rate decreases
OR
slower reaction
«ethanoic acid» partially dissociated/ionized «in solution/water»
OR
lower [H+]
Accept “weak acid” or “higher pH”.
[2 marks]
«pH» converts «wide range of [H+]» into simple «log» scale/numbers
OR
«pH» avoids need for exponential/scientific notation
OR
«pH» converts small numbers into values «typically» between 0/1 and 14
OR
«pH» allows easy comparison of values of [H+]
Accept “uses values between 0/1 and 14”.
Do not accept “easier to use”.
Do not accept “easier for calculations”.
[1 mark]
«species» do not differ by a «single» proton/H+
OR
conjugate base of H3PO4 is H2PO4– «not HPO42–»
OR
conjugate acid of HPO42– is H2PO4– «not H3PO4»
Do not accept “hydrogen/H” for “H+/proton”.
[1 mark]
Examiners report
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the \({}_{22}^{48}{\text{Ti}}\) atom.
State the full electron configuration of the \({}_{22}^{48}{\text{Ti}}\)2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Markscheme
electrostatic attraction
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
Accept mobile electrons.
Do not accept “metal atoms/nuclei”.
[2 marks]
\(\frac{{(46 \times 7.98) + (47 \times 7.32) + (48 \times 73.99) + (49 \times 5.46) + (50 \times 5.25)}}{{100}}\)
= 47.93
Answer must have two decimal places with a value from 47.90 to 48.00.
Award [2] for correct final answer.
Award [0] for 47.87 (data booklet value).
[2 marks]
Protons: 22 AND Neutrons: 26 AND Electrons: 22
[1 mark]
1s22s22p63s23p63d2
[1 mark]
titanium atoms/ions distort the regular arrangement of atoms/ions
OR
titanium atoms/ions are a different size to aluminium «atoms/ions»
prevent layers sliding over each other
Accept diagram showing different sizes of atoms/ions.
[2 marks]
ionic
OR
«electrostatic» attraction between oppositely charged ions
[1 mark]
«simple» molecular structure
OR
weak«er» intermolecular bonds
OR
weak«er» bonds between molecules
Accept specific examples of weak bonds such as London/dispersion and van der Waals.
Do not accept “covalent”.
[1 mark]
TiCl4(l) + 2H2O(l) → TiO2(s) + 4HCl(aq)
correct products
correct balancing
Accept ionic equation.
Award M2 if products are HCl and a compound of Ti and O.
[2 marks]
HCl causes breathing/respiratory problems
OR
HCl is an irritant
OR
HCl is toxic
OR
HCl has acidic vapour
OR
HCl is corrosive
Accept “TiO2 causes breathing problems/is an irritant”.
Accept “harmful” for both HCl and TiO2.
Accept “smoke is asphyxiant”.
[1 mark]
Examiners report
Soluble acids and bases ionize in water.
Sodium hypochlorite ionizes in water.
OCl–(aq) + H2O(l) \( \rightleftharpoons \) OH–(aq) + HOCl(aq)
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with 0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
Identify the amphiprotic species.
Identify one conjugate acid-base pair in the reaction.
Calculate the amount, in mol, of NaOH(aq) used.
Calculate the molar mass of the acid.
Calculate [H+] in the NaOH solution.
Markscheme
water/H2O
Accept “hydroxide ion/OH–”.
[1 mark]
[1 mark]
«0.100 mol\(\,\)dm–3 x 0.0250 dm3» = 0.00250 «mol»
[1 mark]
«M = \(\frac{{0.510{\text{ g}}}}{{0.00250{\text{ mol}}}}\) =» 204 «g\(\,\)mol–1»
[1 mark]
«1.00 x 10–14 = [H+] x 0.100»
1.00 x 10–13 «mol\(\,\)dm–3»
[1 mark]
Examiners report
Water is an important substance that is abundant on the Earth’s surface. Water dissociates according to the following equation.
\[{{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
The graph below shows how the volume of carbon dioxide formed varies with time when a hydrochloric acid solution is added to excess calcium carbonate in a flask.
(i) State the equilibrium constant expression for the dissociation of water.
(ii) Explain why even a very acidic aqueous solution still has some \({\text{O}}{{\text{H}}^ - }\) ions present in it.
(iii) State and explain the effect of increasing temperature on the equilibrium constant above given that the dissociation of water is an endothermic process.
(iv) The pH of a solution is 2. If its pH is increased to 6, deduce how the hydrogen ion concentration changes.
In carbonated drinks containing dissolved carbon dioxide under high pressure, the
following dynamic equilibrium exists.
\[{\text{C}}{{\text{O}}_2}({\text{aq)}} \rightleftharpoons {\text{C}}{{\text{O}}_2}({\text{g)}}\]
Describe the effect of opening a carbonated drink container and outline how this
equilibrium is affected.
(i) Explain the shape of the curve.
(ii) Copy the above graph on your answer sheet and sketch the curve you would obtain if double the volume of hydrochloric acid solution of half the concentration as in the example above is used instead, with all other variables kept constant from the original. Explain why the shape of the curve is different.
(iii) Outline one other way in which the rate of this reaction can be studied in a school laboratory. Sketch a graph to illustrate how the selected variable would change with time.
(iv) Define the term activation energy and state one reason why the reaction between calcium carbonate and hydrochloric acid takes place at a reasonably fast rate at room temperature.
Markscheme
(i) \({K_{\text{c}}} = \frac{{{\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}}}{{{\text{[}}{{\text{H}}_2}{\text{O]}}}}/{K_{\text{c}}} = \frac{{{{{\text{[}}{{\text{H}}_3}{\text{O]}}}^ + }{\text{[O}}{{\text{H}}^ - }{\text{]}}}}{{{\text{[}}{{\text{H}}_2}{\text{O]}}}}/{K_{\text{w}}} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}/{K_{\text{w}}} = {\text{[}}{{\text{H}}_{\text{3}}}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ - }{\text{]}}\);
Do not award mark if [ ] are omitted or other brackets are used.
Expression must be consistent with \({K_{\text{c}}}/{K_{\text{w}}}\).
(ii) \({\text{[}}{{\text{H}}^ + }{\text{]}}\) increases, \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) decreases but still some present (\({K_{\text{w}}}/{K_{\text{c}}}\) constant) / \({\text{[O}}{{\text{H}}^ - }{\text{]}}\)
cannot go to zero as equilibrium present / \({\text{[O}}{{\text{H}}^ - }{\text{]}} = \frac{{{K_{\text{w}}}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}/\frac{{{K_{\text{c}}}{\text{[}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{H}}^ + }{\text{]}}}}\), thus \({\text{[O}}{{\text{H}}^ - }{\text{]}}\)
cannot be zero / OWTTE;
Accept equilibrium present.
(iii) (changing T disturbs equilibrium) forward reaction favoured / equilibrium shifts to the right;
to use up (some of the) heat supplied;
(\({K_{\text{w}}}/{K_{\text{c}}}\)) increases (as both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and \({\text{[O}}{{\text{H}}^ - }{\text{]}}\) increase);
(iv) \({\text{pH}} = 2{\text{, [}}{{\text{H}}^ + }{\text{]}} = 0.01{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{pH}} = 6{\text{, [}}{{\text{H}}^ + }{\text{]}} = {10^{ - 6}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = {10^{ - {\text{pH}}}}\);
\({\text{[}}{{\text{H}}^ + }{\text{]}}\) decreased/changed by \({\text{10000/1}}{{\text{0}}^{ - 4}}\);
Award [2] for correct final answer.
\({\text{C}}{{\text{O}}_2}({\text{g)}}\) /gas escapes / (gas) pressure / \({\text{C}}{{\text{O}}_2}\) (above liquid) decreases / bubbles (of \({\text{C}}{{\text{O}}_2}\) gas) form in the liquid;
equilibrium shifts to the right (to replace the lost \({\text{C}}{{\text{O}}_2}\) gas);
(i) rate = increase in \(\frac{{{\text{volume}}}}{{{\text{time}}}}\) = slope of graph;
initially/to begin with steeper slope / fastest rate / volume of gas/ \({\text{C}}{{\text{O}}_2}\) produced faster/quickly as concentration of HCl highest / OWTTE;
as reaction progresses/with time, less steep slope / volume of gas production slows / rate decreases due to less frequent collisions as concentration (of HCl) decreases / OWTTE;
curve flattens/becomes horizontal when HCl used up/consumed (as there are no more \({{\text{H}}^ + }\) ions to collide with the \({\text{CaC}}{{\text{O}}_3}\) particles);
Each mark requires explanation.
(ii) 
less steep curve;
same maximum volume at later time;
half/lower \({{\text{H}}^ + }\)/acid concentration less frequent collisions slower rate;
same amount of HCl, same volume \({\text{C}}{{\text{O}}_2}\) produced;
(iii) mass loss/of \({\text{C}}{{\text{O}}_2}\) / mass of flask + content;
 ;
;
OR
 ;
;
OR
 ;
;
Do not penalize for missing x-axis label or for missing units on y-axis.
Accept if line meets time axis.
Award [1 max] if temperature is on the vertical axis and magnitude of slope decreases with time.
(iv) minimum/least energy (of colliding particles) for a reaction to occur / OWTTE;
low/lower \({E_{\text{a}}}\) /activation energy / greater/larger surface area/contact between \({\text{CaC}}{{\text{O}}_{\text{3}}}\) and HCl / high/higher HCl concentration/[HCl] / (sufficient) particles/molecules have activation energy;
Examiners report
This was the most popular question in Section B but responses were mixed. Part (a) was generally well dealt with but some candidates confused \({K_{\text{w}}}\) with \({K_{\text{c}}}\) or forgot to include charges on the ions in the equilibrium constant expression. Few received the mark for question (ii) although some mentioned equilibrium which was sufficient.
Candidates recognised that increasing the temperature shifts the equilibrium to the right, but most did not explain why, namely to use up some of the heat supplied. The calculation in (iv) was quite well done although some only gave a qualitative answer.
The equilibrium of carbonated drinks was well understood.
In part (c) (i) candidates frequently described the shape of the curve instead of offering an explanation using collisions theory. Candidates did state, for example, that the curve flattens but did not refer to consumption of HCl(aq), the limiting reagent. Only the better candidates were only able to link slope with rate and some still consider the rate to increase after the reaction has started. In (ii) most realised that the curve would be less steep but few drew a curve with the same maximum volume produced at a later time. Even fewer candidates were able to explain why the number of moles of carbon dioxide remained the same. Although some candidates chose mass loss / pH / pressure as the dependant variable in c(iii), some were penalised for imprecise answers such as mass of reactants without referring to mass of flask. Others misunderstood the question and described experiments that they had done with catalysis or described changes with temperature as the dependant variable. (c)(iv) was generally well answered, but again some responses lacked precision; the activation energy is the minimum energy needed for a reaction to occur.
Consider the following reactions.
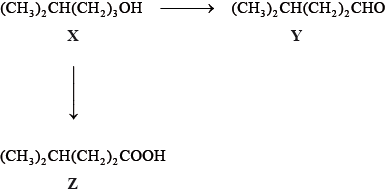
An important environmental consideration is the appropriate disposal of cleaning solvents. An environmental waste treatment company analysed a cleaning solvent, J, and found it to contain the elements carbon, hydrogen and chlorine only. The chemical composition of J was determined using different analytical chemistry techniques.
Combustion Reaction:
Combustion of 1.30 g of J gave 0.872 g \({\text{C}}{{\text{O}}_{\text{2}}}\) and 0.089 g \({{\text{H}}_{\text{2}}}{\text{O}}\).
Precipitation Reaction with AgNO3(aq):
0.535 g of J gave 1.75 g AgCl precipitate.
One example of a homologous series is the alcohols. Describe two features of a homologous series.
The IUPAC name of X is 4-methylpentan-1-ol. State the IUPAC names of Y and Z.
Y:
Z:
State the reagents and reaction conditions used to convert X to Y and X to Z.
X to Y:
X to Z:
Z is an example of a weak acid. State what is meant by the term weak acid.
Discuss the volatility of Y compared to Z.
Determine the percentage by mass of carbon and hydrogen in J, using the combustion data.
Determine the percentage by mass of chlorine in J, using the precipitation data.
The molar mass was determined to be \({\text{131.38 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Deduce the molecular formula of J.
Markscheme
same functional group;
successive/neighbouring members differ by \({\text{C}}{{\text{H}}_{\text{2}}}\);
same general formula;
similar chemical properties;
gradation in physical properties;
Y: 4-methylpentanal;
Z: 4-methylpentanoic acid;
Award [1] if student has correct endings for both molecules but has used incorrect stem.
For both reactions reagents:
named suitable acidified oxidizing agent;
Suitable oxidizing agents are potassium dichromate(VI)/K2Cr2O7 / sodium dichromate(VI)/Na2Cr2O7 / dichromate/Cr2O72– / potassium manganate(VII)/potassium permanganate/KMnO4 / permanganate/manganate(VII)/MnO4–.
Accept H+/H2SO4 instead of sulfuric acid and acidified.
Allow potassium dichromate or sodium dichromate (i.e. without (VI)) or potassium manganate (i.e. without (VII).
Conditions:
distillation for X to Y and reflux for X to Z;
Award [1] if correct reagents and conditions identified for one process only.
acid partially dissociates/ionizes;
Y more volatile than Z;
hydrogen bonding in carboxylic acid/Z;
Accept converse argument.
\(\left( {\left( {\frac{{2 \times 1.01}}{{18.02}}} \right)(0.089) = } \right){\text{ }}1.0 \times {10^{ - 2}}{\text{ g H}}\) and \(\left( {\left( {\frac{{12.01}}{{44.01}}} \right)(0.872) = } \right){\text{ }}2.38 \times {10^{ - 1}}{\text{ g C}}\);
\(\left( {\left( {\frac{{0.238}}{{1.30}}} \right)(100) = } \right){\text{ }}18.3\% {\text{ C}}\);
\(\left( {\frac{{1.0 \times {{10}^{ - 2}}}}{{1.30}}} \right)(100) = 0.77\% {\text{ H}}\);
Award [3] for correct final answer of 18.3% C and 0.77% H without working.
Allow whole numbers for molar masses.
\(\left( {(1.75)\left( {\frac{{35.45}}{{143.32}}} \right) = } \right){\text{ }}0.433{\text{ g (Cl)}}\) and \(\left( {\left( {\frac{{0.433}}{{0.535}}} \right)(100) = } \right){\text{ }}80.9\% {\text{ (Cl)}}\);
Allow whole numbers for molar masses.
\(\left( {\frac{{18.3}}{{12.01}}} \right) = 1.52{\text{ mol C}}\) and \(\left( {\frac{{0.77}}{{1.01}}} \right) = 0.76{\text{ mol H}}\) and \(\left( {\frac{{80.9}}{{35.45}}} \right) = 2.28{\text{ mol Cl}}\);
Allow whole numbers for atomic masses.
Empirical formula \( = {{\text{C}}_2}{\text{HC}}{{\text{l}}_3}\);
Award [2] for correct empirical formula without working.
\({M_{\text{r}}} = (24.02 + 1.01 + 106.35) = 131.38\), so molecular formula is \({{\text{C}}_2}{\text{HC}}{{\text{l}}_3}\);
Award [3] for correct final answer without working.
Allow whole numbers for atomic masses.
Examiners report
Part (a) which asked for a description of a homologous series was generally very well answered.
1 out of 2 marks were commonly awarded, as students had the incorrect prefix or made errors such as 4-methylpentan-1-al instead of 4-methylpentanal.
Most candidates knew the reagents for the conversions of the alcohol but only the best candidates also knew the conditions.
Explanations of a weak acid were well done.
Explanations of volatility were well done.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.
Part (d) was a moles calculation based on experimental data, and was done very well by some of those that attempted it. However many candidates could not get through it and some left it blank.
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Markscheme
n(Ag) = «\(\frac{{3.275{\text{ g}}}}{{107.87{\text{ g}}\,{\text{mol}}}} = \)» 0.03036 «mol»
AND
n(O) = «\(\frac{{3.760{\text{ g}} - 3.275{\text{ g}}}}{{16.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \frac{{0.485}}{{16.00}} = \)» 0.03031 «mol»
«\(\frac{{0.03036}}{{0.03031}} \approx 1\) / ratio of Ag to O approximately 1 : 1, so»
AgO
Accept other valid methods for M1.
Award [1 max] for correct empirical formula if method not shown.
[2 marks]
temperature too low
OR
heating time too short
OR
oxide not decomposed completely
heat sample to constant mass «for three or more trials»
Accept “not heated strongly enough”.
If M1 as per markscheme, M2 can only be awarded for constant mass technique.
Accept "soot deposition" (M1) and any suitable way to reduce it (for M2).
Accept "absorbs moisture from atmosphere" (M1) and "cool in dessicator" (M2).
Award [1 max] for reference to impurity AND design improvement.
[2 marks]
Ar closer to 107/less than 108 «so more 107Ag»
OR
Ar less than the average of (107 + 109) «so more 107Ag»
Accept calculations that gives greater than 50% 107Ag.
[1 mark]
Do not accept name for the products.
Accept “Na+ + OH–” for NaOH.
Ignore coefficients in front of formula.
[3 marks]
«molten» Na2O has mobile ions/charged particles AND conducts electricity
«molten» P4O10 does not have mobile ions/charged particles AND does not conduct electricity/is poor conductor of electricity
Do not award marks without concept of mobile charges being present.
Award [1 max] if type of bonding or electrical conductivity correctly identified in each compound.
Do not accept answers based on electrons.
Award [1 max] if reference made to solution.
[2 marks]
electrons in discrete/specific/certain/different shells/energy levels
energy levels converge/get closer together at higher energies
OR
energy levels converge with distance from the nucleus
Accept appropriate diagram for M1, M2 or both.
Do not give marks for answers that refer to the lines in the spectrum.
[2 marks]
Examiners report
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 g\(\,\)dm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) \( \rightleftharpoons \) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJ\(\,\)mol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJ\(\,\)mol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Markscheme
107°
Accept 100° to < 109.5°.
Literature value = 105.8°
[1 mark]
removes/reacts with OH−
moves to the right/products «to replace OH− ions»
Accept ionic equation for M1.
[2 marks]
N2H4(aq) + H2O(l) \( \rightleftharpoons \) N2H5+(aq) + OH–(aq)
Accept N2H4(aq) + 2H2O(l) \( \rightleftharpoons \) N2H62+(aq) + 2OH–(aq).
Equilibrium sign must be present.
[1 mark]
bubbles
OR
gas
OR
magnesium disappears
2NH4+(aq) + Mg(s) → Mg2+(aq) + 2NH3(aq) + H2(g)
Do not accept “hydrogen” without reference to observed changes.
Accept "smell of ammonia".
Accept 2H+(aq) + Mg(s) → Mg2+(aq) + H2(g)
Equation must be ionic.
[2 mark]
no oxygen required
[1 mark]
bonds broken:
E(N–N) + 4E(N–H)
OR
158 «kJ\(\,\)mol–1» + 4 x 391 «kJ\(\,\)mol–1» / 1722 «kJ»
bonds formed:
E(N≡N) + 2E(H–H)
OR
945 «kJ\(\,\)mol–1» + 2 x 436 «kJ\(\,\)mol–1» / 1817 «kJ»
«ΔH = bonds broken – bonds formed = 1722 – 1817 =» –95 «kJ»
Award [3] for correct final answer.
Award [2 max] for +95 «kJ».
[3 marks]
OR
ΔHvap= −50.6 kJ\(\,\)mol−1 − (−95 kJ\(\,\)mol−1)
«ΔHvap =» +44 «kJ\(\,\)mol−1»
Award [2] for correct final answer.
Award [1 max] for −44 «kJ\(\,\)mol−1».
Award [2] for:
ΔHvap − = 50.6 kJ\(\,\)mol−1 − (−85 kJ\(\,\)mol−1) + = 34 «kJ\(\,\)mol−1».
Award [1 max] for −34 «kJ\(\,\)mol−1».
[2 marks]
total mass of oxygen «= 8.0 x 10–3 g\(\,\)dm–3 x 1000 dm3» = 8.0 «g»
n(O2) «\( = \frac{{8.0{\text{ g}}}}{{32.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \)» 0.25 «mol»
OR
n(N2H4) = n(O2)
«mass of hydrazine = 0.25 mol x 32.06 g\(\,\)mol–1 =» 8.0 «g»
Award [3] for correct final answer.
[3 marks]
«n(N2H4) = n(O2) \( = \frac{{8.0{\text{ g}}}}{{32.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \)» 0.25 «mol»
«volume of nitrogen = 0.25 mol x 24.8 dm3\(\,\)mol–1» = 6.2 «dm3»
Award [1] for correct final answer.
[1 mark]
Examiners report
Magnesium reacts with sulfuric acid:
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
The graph shows the results of an experiment using excess magnesium ribbon and dilute sulfuric acid.
Outline why the rate of the reaction decreases with time.
Sketch, on the same graph, the expected results if the experiment were repeated using powdered magnesium, keeping its mass and all other variables unchanged.
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) \( \rightleftharpoons \) NO(g) + CO2(g) ΔH = –226 kJ
Calculate the activation energy for the reverse reaction.
State the equation for the reaction of NO2 in the atmosphere to produce acid deposition.
Markscheme
concentration of acid decreases
OR
surface area of magnesium decreases
Accept “less frequency/chance/rate/probability/likelihood of collisions”.
Do not accept just “less acid” or “less magnesium”.
Do not accept “concentrations of reagents decrease”.
[1 mark]
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
«Ea(rev) = 226 + 132 =» 358 «kJ»
Do not accept –358.
[1 mark]
2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
OR
2NO2(g) + 2H2O(l) + O2(g) → 4HNO3(aq)
Accept ionised forms of the acids.
[1 mark]
Examiners report
Many reactions are in a state of equilibrium.
The equations for two acid-base reactions are given below.
HCO3– (aq) + H2O (l) \( \rightleftharpoons \) H2CO3 (aq) + OH– (aq)
HCO3– (aq) + H2O (l) \( \rightleftharpoons \) CO32– (aq) + H3O+ (aq)
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) \( \rightleftharpoons \) 2HI (g) ΔHθ < 0
Outline the effect, if any, of each of the following changes on the position of equilibrium, giving a reason in each case.
Identify two different amphiprotic species in the above reactions.
State what is meant by the term conjugate base.
State the conjugate base of the hydroxide ion, OH–.
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
Markscheme
Award [1 max] if both effects are correct.
Reason for increasing volume:
Accept “concentration of all reagents reduced by an equal amount so cancels out in Kc expression”.
Accept “affects both forward and backward rates equally”.
HCO3– AND H2O
species that has one less proton/H+ ion «than its conjugate acid»
OR
species that forms its conjugate acid by accepting a proton
OR
species that is formed when an acid donates a proton
Do not accept “differs by one proton/H+ from conjugate acid”.
oxide ion/O2–
insufficient data to make generalization
OR
need to consider a «much» larger number of acids
OR
hypothesis will continue to be tested with new acids to see if it can stand the test of time
«hypothesis is false as» other acids/HCl/HBr/HCN/transition metal ion/BF3 do not contain oxygen
OR
other acids/HCl/HBr/HCN/transition metal ion/BF3 falsify hypothesis
correct inductive reasoning «based on limited sample»
«hypothesis not valid as» it contradicts current/accepted theories/Brønsted-Lowry/Lewis theory
[Max 2 Marks]
Examiners report
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
Identify the formula and state symbol of X.
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
The precipitate of sulfur makes the mixture cloudy, so a mark underneath the reaction mixture becomes invisible with time.
10.0 cm3 of 2.00 mol dm-3 hydrochloric acid was added to a 50.0 cm3 solution of sodium thiosulfate at temperature, T1. Students measured the time taken for the mark to be no longer visible to the naked eye. The experiment was repeated at different concentrations of sodium thiosulfate.
Show that the hydrochloric acid added to the flask in experiment 1 is in excess.
Draw the best fit line of \(\frac{1}{{\rm{t}}}\) against concentration of sodium thiosulfate on the axes provided.
A student decided to carry out another experiment using 0.075 mol dm-3 solution of sodium thiosulfate under the same conditions. Determine the time taken for the mark to be no longer visible.
An additional experiment was carried out at a higher temperature, T2.
(i) On the same axes, sketch Maxwell–Boltzmann energy distribution curves at the two temperatures T1 and T2, where T2 > T1.
(ii) Explain why a higher temperature causes the rate of reaction to increase.
Suggest one reason why the values of rates of reactions obtained at higher temperatures may be less accurate.
Markscheme
H2O AND (l)
Do not accept H2O (aq).
SO2 (g) is an irritant/causes breathing problems
OR
SO2 (g) is poisonous/toxic
Accept SO2 (g) is acidic, but do not accept “causes acid rain”.
Accept SO2 (g) is harmful.
Accept SO2 (g) has a foul/pungent smell.
n(HCl) = «\(\frac{{10.0}}{{1000}}\)dm3 × 2.00 mol dm-3 =» 0.0200 / 2.00 × 10-2«mol»
AND
n(Na2S2O3) = «\(\frac{{50}}{{1000}}\)dm3 × 0.150 mol × dm-3 =» 0.00750 / 7.50 × 10-3 «mol»
0.0200 «mol» > 0.0150 «mol»
OR
2.00 × 10-2«mol» > 2 × 7.50 × 10-3 «mol»
OR
\(\frac{1}{2}\) × 2.00 × 10-2 «mol» > 7.50 × 10-3 «mol»
Accept answers based on volume of solutions required for complete reaction.
Award [2] for second marking point.
Do not award M2 unless factor of 2 (or half) is used.
five points plotted correctly
best fit line drawn with ruler, going through the origin
22.5 × 10-3 «s-1»
«Time = \(\frac{1}{{22.5 \times {{10}^{ - 3}}}}\) =» 44.4 «s»
Award [2] for correct final answer.
Accept value based on candidate’s graph.
Award M2 as ECF from M1.
Award [1 max] for methods involving taking mean of appropriate pairs of \(\frac{1}{{\rm{t}}}\) values.
Award [0] for taking mean of pairs of time values.
Award [2] for answers between 42.4 and 46.4 «s».
(i)
correctly labelled axes
peak of T2 curve lower AND to the right of T1 curve
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/EK” but not just “Energy/E” on x-axis.
(ii)
greater proportion of molecules have E ≥ Ea or E > Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Accept more molecules have energy greater than Ea.
Do not accept just “particles have greater kinetic energy”.
Accept “rate/chance/probability/likelihood/” instead of “frequency”.
Accept suitably shaded/annotated diagram.
Do not accept just “more collisions”.
shorter reaction time so larger «%» error in timing/seeing when mark disappears
Accept cooling of reaction mixture during course of reaction.