Question 19M.2.hl.TZ2.1c(ii)
| Date | May 2019 | Marks available | [Maximum mark: 3] | Reference code | 19M.2.hl.TZ2.1c(ii) |
| Level | hl | Paper | 2 | Time zone | TZ2 |
| Command term | Determine | Question number | c(ii) | Adapted from | N/A |
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
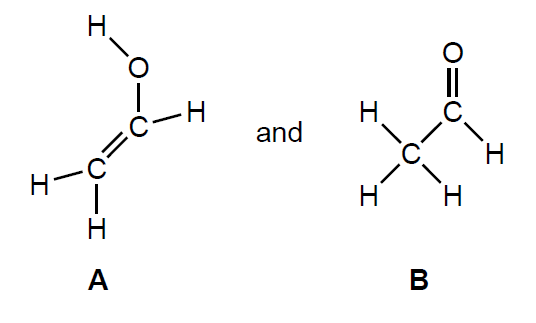
Two possible products are:
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
[3]
«sum of bond enthalpies of reactants =» 2(C—H)+C ≡ C + 2(O—H)
OR
2 × 414 «kJ mol-1» + 839 «kJ mol-1» + 2 × 463 «kJ mol-1»
OR
2593 «kJ» [✔]
«sum of bond enthalpies of A =» 3(C—H) + C=C + C—O + O—H
OR
3 × 414 «kJ mol-1» + 614 «kJ mol-1» + 358 «kJ mol-1» + 463 «kJ mol-1»
OR
2677 «kJ» [✔]
«enthalpy of reaction = 2593 kJ – 2677 kJ» = –84 «kJ» [✔]
Note: Award [3] for correct final answer.
Candidates were able to calculate the ΔH of the given reaction correctly; a few inverted the calculations or made mathematical errors.