Question 19M.2.hl.TZ2.1c(iv)
| Date | May 2019 | Marks available | [Maximum mark: 2] | Reference code | 19M.2.hl.TZ2.1c(iv) |
| Level | hl | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | c(iv) | Adapted from | N/A |
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
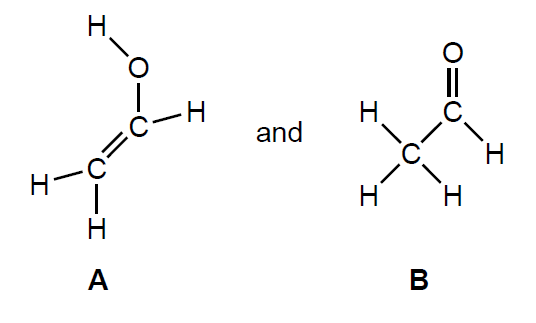
Two possible products are:
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
[2]
Identity of product: «B»
IR spectrum:
1700–1750 «cm–1 band» AND carbonyl/CO group present
OR
no «band at» 1620–1680 «cm–1» AND absence of double bond/C=C
OR
no «broad band at» 3200–3600 «cm–1 » AND absence of hydroxyl/OH group [✔]
1H NMR spectrum:
«only» two signals AND A would have three
OR
«signal at» 9.4–10.0 «ppm» AND «H atom/proton of» aldehyde/–CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of alkyl/CH next to» aldehyde/CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of» RCOCH2- present
OR
no «signal at» 4.5–6.0 «ppm» AND absence of «H atom/proton next to» double bond/C=C ✔
Note: Accept a specific value or range of wavenumbers and chemical shifts.
Accept “two signals with areas 1:3”.
Interpretation of spectra was very good and the few candidates that lost marks with 1H NMR data rather than IR, for example simply mentioning two signals for B. However, most candidates that attempted this question got full marks.