Some thoughts on the May 2022 IB Chemistry examinations
Monday 30 May 2022
Questions with more than one 'best' answer
About a year ago I wrote a blog Thinking through multiple choice questions on the difficulty of writing good multiple choice questions. This is partly due to the fact that because the numbers of words should be kept to a minimum it is a challenge to cover all the parameters so that only one of the four answers can be correct (or at least much better than any of the other three possible answers). This seems to be the case with one of the questions that has appeared on this year’s May multiple choice papers.
Question 28 (SL) and 38 (HL) asks how many signals would be observed in the 1H NMR spectrum of a compound for which no name is given but the structure is shown.
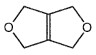
The molecular formula is C6H8O2 and all eight hydrogen atoms are in the same chemical environment so students should fairly easily be able to see that it will give one signal. The problem is that the question does not actually ask how many signals the compound will give – it asks how many will be observed. 1H NMR spectra need a reference so that the chemical shift can be measured in ppm. This reference is often tetramethylsilane Si(CH3)4 which gives a strong single signal at 0 ppm due to the 12 equivalent hydrogen atoms so actually two signals may well be observed, one from the compound and one from the reference. I hope the IB will consider giving both 1 and 2 as the correct answer. Of course, this could easily have been clarified in the wording of the question either by asking how many signals will the compound give in its 1H NMR spectrum or alternatively by adding “ignore the signal due to the reference”.
(Incidentally, I think it is bad practice not to name a compound in a question but I must admit that I am having trouble naming it and cannot actually find either the compound or its 1H NMR spectrum online – maybe someone can enlighten me? - Update someone has! I’m grateful to Adrian Dingle who has identified it using ChemDoodle as 3,7-dioxabicyclo[3.3.0]oct-1(5)-ene. In fact the first report of its synthesis by C. Mlynek et al. was in 2005 in the Journal of Molecular Structure.)
I think there are also issues with another question on the HL paper.
Question 21 asks which factor influences the pre-exponential factor, A in the Arrhenius equation and the students can choose from A. Nature of reactants, B. Temperature of reaction, C. Activation energy of reaction and D. Overall order of reaction.
I’ve shared my thoughts about this question with three very experienced IB teachers and like me they all feel that it seems way too hard for IB students to answer in a multiple choice question, where they cannot explain their single answer, as there are multiple answers. The question does not include “in a specified reaction” so anything that might alter the value of A in the general equation could be considered correct. Clearly different reactions will have different values of A so it depends upon the nature of the reactants. The temperature dependence of the reaction is usually accounted for in the Ea/RT exponential part of the equation but A refers to the orientation of the successful collisions and at higher temperatures there will be more collisions so it is generally accepted that A is also temperature dependent. The activation energy of a reaction can be lowered by adding a catalyst which provides a different pathway, this will also affect the mechanism and hence the orientation of how the reactants collide so affecting the value of A. Finally A has the same units as the rate constant, k. The overall order of the reaction determines the units of the rate constant so therefore changing the overall order of the reaction will also change the value of A, as its units change.
... and some pedantry!
There are also a few pedantic points which I’m sure will not have affected how the students performed on the exams but the proof reading does not appear to have been very thorough which mars the overall professionalism and quality of the papers.
There are inconsistencies in the use of the language of chemistry. Several of the questions contain constants in normal lettering and (correctly) in italics in different questions and even in the same question, e.g. A and A in Question 21 discussed above in HLP1 and Question 13 in SLP1 has ΔH in answers A, B and D but ΔH in answer C. Similarly Question 30 has the E in E⦵ not in italics whereas in Question 3(a)(i) in HL Paper 2 it is shown correctly with the E in E⦵ In italics. There is confusion between the use of “what” and “which” in the multiple choice questions (e.g. compare Questions 26 and 29 on the SL Paper 1). In the past the rule followed was In general, use "which" before a noun and "what" before a verb. Now the choice of “which” or “what” seems random. The IB has for the past few years asked for “calculate the amount (in mol)” and this year in Question 1 on Paper 2 has reverted back to “calculate the number of moles” - perhaps because this is how it was worded in Question 2 on the June 2014 Cambridge International AS examination from which the question appears to have been taken from?



Comments 26
Geoff,
The NMR question is irksome at best and I would be unhappy if the IB don't grant the kids some latitude with responses accepted.
The Arrhenius question is pretty impenetrable for the majority of students and perplexes even experienced teachers. I cant imagine how this passed Quality Assurance checks. It clearly did so maybe the IB QA systems need some reviewing.
Copying a question verbatim is reprehensible and reduces the credibility of the IB zero tolerance stance on copying significantly.
And I love a bit of pedantry !
Warm Regards
James ( Mr M 4 Chem )
I agree with James here that copying whole questions from previous A-level papers is unacceptable - what if a student was somehow to find that question before the exam? Wouldn't that give the student an unfair advantage? And considering the IB's strict policy on academic honesty - how does it look when they are copying questions from previous exam papers?
Regarding the HNMR question, it bothers me that the IB often omits the TMS peak from spectra when given in exams (such as the November 2021 paper 1 question on HNMR). The other points are also valid, I just wonder if anyone higher up in the exam making process will read this (perhaps if I share on MYIB to give it some traction)?
So I've linked to this on MyIB together with a side by side comparison of the two questions. Another thing I noticed is that they are not even being consistent with "number of moles" vs "amount" in the same question. Parts (b) and (c) have "calculate the number of moles" whereas part (d) has "calculate the amount".
I have shared the Arrhenius question with my DP 1 and they discussed it as a group and came up with this conclusion; all answers are correct under different conditions, so more data/information needed in the question. Thank you all for taking time and reporting these frustrating facts. I hope IB somewhat fixes these issues and give credit to students.
Question 17 in HL P1: I guess they consider B as the correct answer but actually ΔS of the surroundings has the opposite sign of ΔΗ of the system, so a minus sign is missing here.
For Qu. 29 in SL P1 there is a discussion in MyIB as the 1/2 of the minimum division is not among the answers.
Hmm. I have to agree on Qu 28/38, so I'll be interested in how they mark it but I'm sure most will not include TMS in their count (as similar practice questions do not include TMS normally as part of the 'count') - the smarter students however ....... AN ISSUE! They have to allow 1 or 2 given the context of the question.
As for Qu 21, with A being a collision frequency / steric factor, I expect my students to be somewhat confused. Although I teach that temp. is accommodated by 'T' (and is less important), and answer 'C' as there as EA, they will no doubt be torn between reactants (A), rds and therefore (D) order of reaction.
Lastly, I'm a stickler for consistency, and strive to be Mr. Pedantic with all I produce in terms of exams, worksheets and with students use of language, knowledge of definitions, etc., especially as most are EFL. This is very poor form, in an exam, in my book. That said I try to introduce the complications of non-standardization as a common 'weakness' in scientific teaching, texts, university lecturing, life!!
So questions need to be asked as to the level of professional review on this paper.
Hi Anthoula,
In my opinion SL Question 29 is a good example of where the IB has failed to take on board international mindedness in chemistry. Generally the IB is well aware that different cultures use different terminologies and have different slants on chemistry. It makes clear the terminology it will use in its questions but also makes clear that students using different correct (and consistent) terminology in submitted work (e.g. their IA or EE) will not be penalised. (For example, “a buret reading of 23.00 mL of 1.00 M acetic acid.” instead of “a burette reading of 23.00 cm3 of 1.00 mol dm–3 ethanoic acid.”) The problem comes with uncertainties. The IB wants students to be aware of uncertainties and to be able to calculate overall uncertainties but, unlike with units and names etc., does not specify how they should be determined. This is fine for submitted student work where any recognised method is acceptable but causes serious problems when setting multiple choice questions where students are unable to show how they have arrived at their answer. Different cultures determine uncertainties in different ways. Question 29 is probably about a graduated measuring cylinder reading. More accurate volumes can be obtained with a burette or graduated pipette so students tend to learn how to record the data when the smallest marked division is 0.1 cm3 (or 0.1 mL) rather than 1.0 cm3 as in Question 29 but the principle is the same. In AP chemistry students read the volume on a buret to two decimal places where the last number can be any number between 0 and 9, i.e. to ± 0.01 cm3 . (For example, AP Chemistry 2018 Free-response question 3(e): “The student titrates a 10.0 mL sample of Fe2+(aq) solution. Calculate the value of [Fe2+] in the solution if it takes 17.48 mL of added 0.0350 M KMnO4(aq) to reach the equivalence point of the titration.”) In A level students also give a burette reading to two decimal places but the last figure can only be 0 or 5, i.e. to ± 0.05 cm3. (For example, OCR A level June 2020 question 4(a): “The burette readings have been recorded to the nearest 0.05cm3. Initial reading 0.05 cm3, final reading 27.80 cm3 etc.".) This explains why some teachers feel that B (± 0.1 cm3) is the correct answer to Question 29 whereas other teachers feel that no correct answer has been given as it should be ± 0.5 cm3. I think the discussion at this year’s Grade Award meeting is going to be interesting!
Dear Geoff,
I agree with your comments on the particular question as well as with the general comment about the challenges of constructing meaningful and concise MC questions.
What do you think about qu. 17? other than the mistake, I think it is also closer to the approach most A level boards have in examining this topic. Although I always teach the spontaneity requirement starting from ΔSuniv>0, I feel that except for the guidance "ΔG is a convenient way to take into account both the direct entropy change resulting from the transformation of the chemicals, and the indirect entropy change of the surroundings as a result of the gain/loss of heat energy" (on page 85 of the guide), not much is said about relating the entropy change of the surroundings to the enthalpy change of the system.
Sorry Anthi, I forgot to also comment on Qu.17 when I replied earlier.
When a chemical reaction takes place at constant pressure with enthalpy change ΔH, the entropy change of the surroundings, ΔSsur = – ΔH/Tsur. Students could perhaps deduce this to answer the question by using units since the units of ΔH are J mol–1 (although normally kJ rather than J are used), the units of temperature are K so dividing the units of kJ mol–1 by K gives the units of ΔS, J K–1 mol–1. As you say there is no correct answer to the question as answer B should be – ΔH not ΔH. As far as I can recollect, no questions on ΔSsur = – ΔH/Tsur have ever been asked before by the IB. It is not spelt out on the syllabus and the IB endorsed OUP Course Companion text book does not include it (neither does my Study Guide).
Marking Scheme says C is the right answer.
Subject report give this feedback/ explanation.
"This question generated a lot of debate among teachers and examiners. The answer B is the best answer but it is a rather low value. It was selected by 34% of the candidates. Many teachers argued that the correct answer should be 0.5 cm3. "
I'm not even sure how I'm going to explain this to my students!!
Oops, pls ignore this comment posted below wrongly for another Q.
Marking Scheme says C is the right answer.
Subject report give this feedback/ explanation.
"This question generated a lot of debate among teachers and examiners. The answer B is the best answer but it is a rather low value. It was selected by 34% of the candidates. Many teachers argued that the correct answer should be 0.5 cm3. "
I'm not even sure how I'm going to explain this to my students!!
Hello , thanks to all the above observations …didn't realize there were so many aspects I missed out in the QP. It was really interesting to read the comments. Thanks Mr. Geoffrey for the deep dive.
I have one query - Q1 From paper 2, 'Complete the ionic equation' - which means the students are expected to know the products to balance the equation. Usually IB doesn't expect students to memorize the equations. The student could have written Mn4+ instead of Mn2+ presuming it to be right as it is getting reduced. I know the equations is given in the redox but jut wondering if they had to remember it ....
I think that technically you are correct Vinaya as it does say in the guide “Deduction of chemical equations when reactants and products are specified” but I would have thought that almost all students will have come across the KMnO4 / Mn2+ redox equation during the two years they have been studying the course and almost all will have seen the colour change to the colourless (actually very pale pink) Mn2+(aq) ion. As you say, the half-equation is also given in Section 24 of the data booklet which students have access to for Paper 2. If you are worried about this you should include it on your Teacher Feedback form. If any student did show it going to Mn(IV) rather than Mn(II) then I would hope that ECF would be applied for the rest of the question.
thanks Mr. Geoffrey.
Qu29. I'm unclear on the discussion above.
For me it is straight forward. For a measuring cylinder I teach students they read it twice when determining the value. With gradations of 0.1cm3 you can read 1/2 way so the error for each is 0.05cm3. Read twice that's 0.05 + 0.05 = 0.1cm3, so the answer is B. All students should be taught that 1 cm3 = 1mL = 1cc, etc., i.e. international mindedness!
What am I missing here?
HL P1, Q17. Surely "indirect measure" allows for "ΔH" rather than "-ΔH"?
Stephen, Yes, of course, all students should be taught that 1cm3 = 1 ml. The IB simply says that it is going to use cm3 and mol dm–3 etc. in questions but is clear that it will accept mL and M etc. in students’ written work. The international mindedness point I was trying to make is the one you have illustrated yourself. You say “For me it is straightforward” but it is not the same for many other teachers from different cultures. If you look at the AP question I quoted it illustrates that those doing AP are not taught the way you are teaching. They will give the second decimal place to a number between 0 and 9 when reading a burette whereas you will only give either 0 or 5 – maybe in the USA etc. they just have better eyesight!
B has to be the “best’ answer of the four possible answers given for Q17 but it does matter hugely whether the value for ΔS is positive or negative and – ΔH/T is “indirectly’ giving the correct value and correct sign for ΔS.
However these are only my views and it is up to the Grade Awarding team (who look at the Teacher Feedback forms) to decide what they think is best, both when it comes to awarding the grades and to writing the subject report afterwards.
OK. I'm not totally convinced that Q17 warrants as much concern, but I am new to IB and have much to appreciate internationally - and I was certainly not aware of the AP point so thank you for that. But, it's clearly tough writing IB questions although, as other questions illustrate, it can be done better.
Hello All
Q29. SL P1. The instrument is a measuring cylinder. An analogue device. The smallest scale division is 1 cm3. The candidate is asked for the uncertainty of this measurement. Just one measurement. Uncertainty should be +/- 0.5% according to most accepted rules for analogue devices (half the smallest scale division}. This was not one of the options.
Hello Christopher, I agree with you.
Agree with Christopher here - half the smallest scale division for analogue apparatus. But this highlights the larger problem of learning things 'the IB way' i.e what the IB accepts as being correct. And I've always thought the IB is overly picky about uncertainties, especially when it comes to the IA. I mean they are important but do they really make that much of a difference in a high school lab?
I agree with Mike, at a level where they have to learn chemistry and basic scientific approaches, they get intimidated by a very strict uncertainty scheme.
Agree entirely. My students spend an inordinate amount of time on their IA work worrying about processing uncertainties correctly at the expense of the actual science. Hope this changes with the new syllabus
On HL paper 2, both question 8d and 8e ask students to describe the splitting patterns of H NMR signals. In both case, the splitting patterns include answers beyond quartet, which the IB specifically says on the syllabus is the maximum splitting pattern students are responsible for knowing. Why is the IB asking about things it explicitly says it will not ask about? I realize it is fairly simple to use the n+1 rule to determine the splitting patterns as quintet and sextet, but if students haven't practiced these examples, there is a good chance they would not use the the correct terminology to describe the splitting. I hope the examiners were lenient in their marking of these 2 questions.
Hi Peter,
Question 8(d) actually asks for two differences in the 1H NMR spectra of but-2-ene and 2-bromobutane, so could be answered by talking about the different number of signals and the different chemical shifts without going into the different splitting patterns. However question 8(e) does specifically ask students to deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane. This is definitely not on the syllabus, should not have been asked and should have been picked up by the external checker before the paper was finalised. The IB should accept 'complex' for the central carbon atom as there are five hydrogen atoms on the adjacent carbon atoms of the –CH2– group resulting in a sextet in the ratio of 1:5:10:10:5:1 which students are not expected to be able to deduce.
It seems that the May 2022 exam question that was copied from the A-level question has been removed from the exam paper available for purchase from the IB website and replaced with a question about lithium and its reaction with water. Does anyone know why?
To post comments you need to log in. If it is your first time you will need to subscribe.