Question 19M.2.hl.TZ2.1
| Date | May 2019 | Marks available | [Maximum mark: 19] | Reference code | 19M.2.hl.TZ2.1 |
| Level | hl | Paper | 2 | Time zone | TZ2 |
| Command term | Compare, Deduce, Determine, Explain, Identify, Predict, State, Suggest, Write | Question number | 1 | Adapted from | N/A |
Ethyne, C2H2, reacts with oxygen in welding torches.
Write an equation for the complete combustion of ethyne.
[1]
C2H2 (g) + 2.5O2 (g) → 2CO2 (g) + H2O (l)
OR
2C2H2 (g) + 5O2 (g) → 4CO2 (g) + 2H2O (l) [✔]
All candidates were able to write the correct reactants/products for combustion of ethyne, but a few failed to balance correctly.

Deduce the Lewis (electron dot) structure of ethyne.
[1]
[✔]
Note: Accept any valid combination of lines, dots and crosses.
Most drew correct Lewis structures for ethyne, though some drew ethene.

Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
[1]
«ethyne» shorter AND a greater number of shared/bonding electrons
OR
«ethyne» shorter AND stronger bond [✔]
Surprisingly very few explained the difference in bond length/strength looking at electrons shared and just gave the shorter/triple or longer/single bond answer.

Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
[1]
London/dispersion/instantaneous dipole-induced dipole forces [✔]
Good to see that most candidates identified the specific IMF correctly.

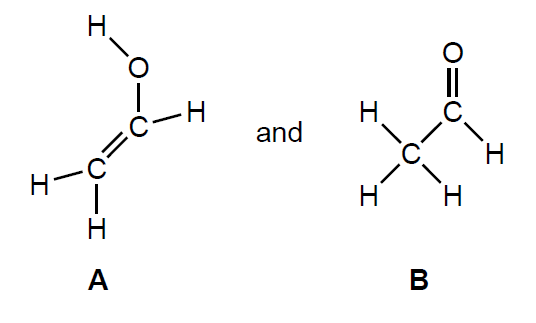
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
State the name of product B, applying IUPAC rules.
[1]
ethanal [✔]
Most candidates gave the correct IUPAC name.

Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
[3]
«sum of bond enthalpies of reactants =» 2(C—H)+C ≡ C + 2(O—H)
OR
2 × 414 «kJ mol-1» + 839 «kJ mol-1» + 2 × 463 «kJ mol-1»
OR
2593 «kJ» [✔]
«sum of bond enthalpies of A =» 3(C—H) + C=C + C—O + O—H
OR
3 × 414 «kJ mol-1» + 614 «kJ mol-1» + 358 «kJ mol-1» + 463 «kJ mol-1»
OR
2677 «kJ» [✔]
«enthalpy of reaction = 2593 kJ – 2677 kJ» = –84 «kJ» [✔]
Note: Award [3] for correct final answer.
Candidates were able to calculate the ΔH of the given reaction correctly; a few inverted the calculations or made mathematical errors.

The enthalpy change for the reaction to produce B is −213 kJ.
Predict, giving a reason, which product is the most stable.
[1]
B AND it has a more negative/lower enthalpy/«potential» energy
OR
B AND more exothermic «enthalpy of reaction from same starting point» [✔]
Generally well done, most common error was stating that the enthalpy change was “larger” without the indication that it was an exothermic change or the sign.

The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
[2]
Identity of product: «B»
IR spectrum:
1700–1750 «cm–1 band» AND carbonyl/CO group present
OR
no «band at» 1620–1680 «cm–1» AND absence of double bond/C=C
OR
no «broad band at» 3200–3600 «cm–1 » AND absence of hydroxyl/OH group [✔]
1H NMR spectrum:
«only» two signals AND A would have three
OR
«signal at» 9.4–10.0 «ppm» AND «H atom/proton of» aldehyde/–CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of alkyl/CH next to» aldehyde/CHO present
OR
«signal at» 2.2–2.7 «ppm» AND «H atom/proton of» RCOCH2- present
OR
no «signal at» 4.5–6.0 «ppm» AND absence of «H atom/proton next to» double bond/C=C ✔
Note: Accept a specific value or range of wavenumbers and chemical shifts.
Accept “two signals with areas 1:3”.
Interpretation of spectra was very good and the few candidates that lost marks with 1H NMR data rather than IR, for example simply mentioning two signals for B. However, most candidates that attempted this question got full marks.

Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
[2]
2.3 ppm: doublet [✔]
9.8 ppm: quartet [✔]
The stronger candidates were able to predict the splitting pattern correctly, others inverted the answer, but many others repeated the information for protons with the given chemical shift, which is unexpected since wording was straightforward.

Product B, CH3CHO, can also be synthesized from ethanol.
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
[2]
Reagents:
acidified/H+ AND «potassium» dichromate«(VI)»/K2Cr2O7/Cr2O72- [✔]
Conditions:
distil «the product before further oxidation» [✔]
Note: Accept “«acidified potassium» manganate(VII)/KMnO4/MnO4-/permanganate”.
Accept “H2SO4” or “H3PO4” for “H+”.
Accept “more dilute dichromate(VI)/manganate(VII)” or “excess ethanol”.
Award M1 if correct reagents given under “Conditions”.
Candidates seemed to be confused by the prompts, reagent and conditions, so often included the acid among conditions. Careless errors were common such as the wrong charge on the dichromate ion. Few candidates suggest permanganate as an option.

Deduce the average oxidation state of carbon in product B.
[1]
–1 [✔]
Most candidates were able to calculate oxidation state of carbon in B.

Explain why product B is water soluble.
[3]
Any three of:
has an oxygen/O atom with a lone pair [✔]
that can form hydrogen bonds/H-bonds «with water molecules» [✔]
hydrocarbon chain is short «so does not disrupt many H-bonds with water molecules» [✔]
«large permanent» dipole-dipole interactions with water [✔]
Candidates did not understand that they must mention the IMF responsible for the solubility. Most candidates explained the polarity of the aldehyde and water but did not mention that this results in permanent dipole-dipole interactions; many did mention H-bonding. The mention of the lone pair on O atom and short hydrocarbon chain were very rare.