Alcohol in the body
Thursday 19 May 2011
Many people’s jobs (e.g. health workers and airline pilots) depend on the fact that they abstain from taking alcohol. The chemistry behind the common methods used to determine the amount of alcohol in the blood, urine and breath are covered in Option D: Medicines and drugs under Assessment statement D.4.3 . These include the reduction of the orange dichromate(VI) ion to the green chromium(III) ion used in the ‘blow in the bag’ and the use of infrared and fuel cells in different types of intoximeters.
 The amount of alcohol present in alcoholic drinks is usually listed clearly on the label on the can or bottle. However alcohol can be imbibed unintentionally in other ways apart from drinking alcoholic drinks. For example, certain mouth washes, gripe water for infants (see left), hand washing gels, hairsprays and cosmetics can all contain ethanol. All of these can boost the amount of alcohol in the body and lead to positive results even if the person in question has not been drinking alcohol as such. Researchers at the University of Florida have been looking at another way of determining alcohol body content by examining the breakdown products in urine. The two breakdown products in particular are ethyl glucuronide (EtG) and ethyl sulfate (EtS).
The amount of alcohol present in alcoholic drinks is usually listed clearly on the label on the can or bottle. However alcohol can be imbibed unintentionally in other ways apart from drinking alcoholic drinks. For example, certain mouth washes, gripe water for infants (see left), hand washing gels, hairsprays and cosmetics can all contain ethanol. All of these can boost the amount of alcohol in the body and lead to positive results even if the person in question has not been drinking alcohol as such. Researchers at the University of Florida have been looking at another way of determining alcohol body content by examining the breakdown products in urine. The two breakdown products in particular are ethyl glucuronide (EtG) and ethyl sulfate (EtS). 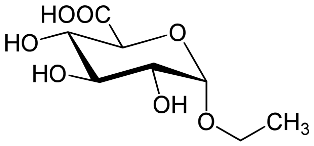 They have shown that non-drinkers who clean their hands regularly using hand sanitisers showed levels of ethyl glucuronide in their urine consistent with alcohol drinkers. However the level of ethyl sulfate was considerably lower. They hope this difference in the levels of the two chemicals may lead to a more reliable test to distinguish between those who have imbibed alcohol and those who have absorbed it through their skin and thus prevent false positive tests and the potentially very damaging wrongful charge of alcohol abuse.
They have shown that non-drinkers who clean their hands regularly using hand sanitisers showed levels of ethyl glucuronide in their urine consistent with alcohol drinkers. However the level of ethyl sulfate was considerably lower. They hope this difference in the levels of the two chemicals may lead to a more reliable test to distinguish between those who have imbibed alcohol and those who have absorbed it through their skin and thus prevent false positive tests and the potentially very damaging wrongful charge of alcohol abuse.
A video ![]() detailing this has been produced by the University of Florida.
detailing this has been produced by the University of Florida.


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.