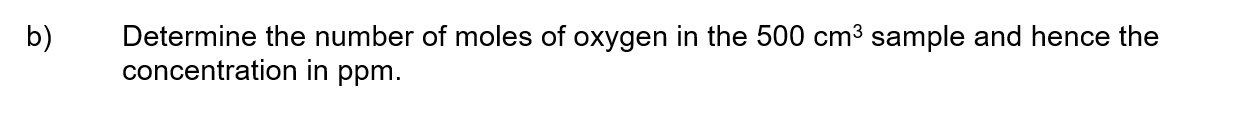Assess your score
View Answer
Assess your score
View Answer
Assess your score
View Answer
Assess your score
View Answer
Next Question
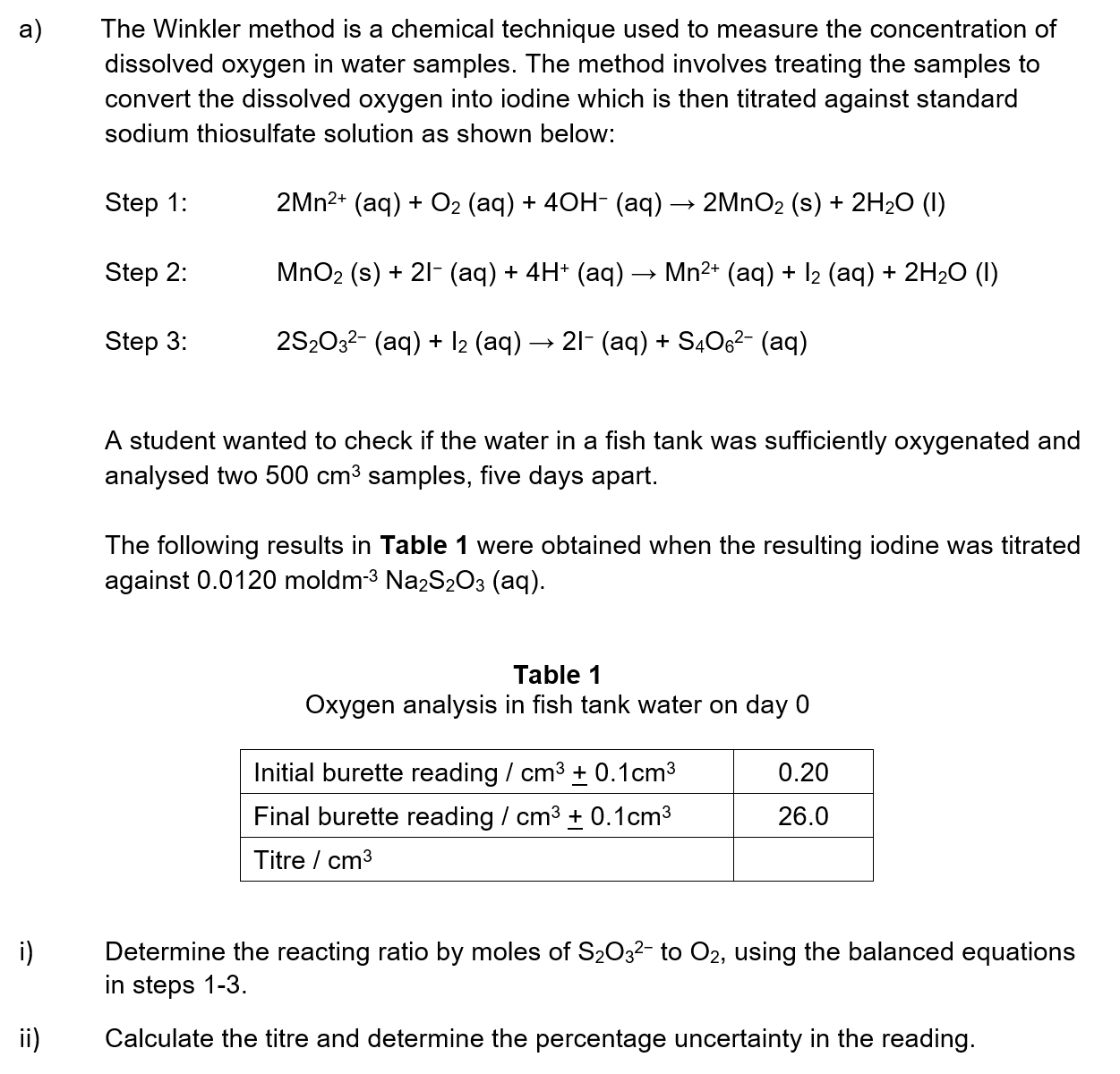
a)
The successive ionisation energies of vanadium are shown.
Assess your score
View Answer
b)
Outline why there is an increase in ionisation energy from electron 3 to electron 5.
Assess your score
View Answer
c)
Explain why there is a large increase in the ionisation energy between electrons 5 and 6.
Assess your score
View Answer
d)
The first six ionisation energies, in kJ mol-1 , of an element are shown below
IE 1
IE 2
IE 3
IE 4
IE 5
IE 6
578
1816
2744
11576
14829
18375
Explain the large increase in ionisation energy from
IE 3 to
IE 4
Assess your score
View Answer
Previous Question Next Question
Assess your score
View Answer
Assess your score
View Answer
Assess your score
View Answer
Previous Question Next Question
a)
When chromium(III) sulfate dissolves in water, a green solution containing the [Cr(H 2 O) 6 ] 3+ ion forms.
i)
State the bond angles found in this complex ion.
[1]
ii)
Explain why the chromium(III) complex ion is coloured.
[3]
Assess your score
View Answer
b)
Vanadium(V) oxide is the catalyst used in the Contact process as shown by the reactions:
SO 2 (g) + V 2 O 5 (s) → SO 3 (g) + V 2 O 4 (s)
V 2 O 4 (s) + ½O 2 (g) → V 2 O 5 (s)
i) Explain, using the equations, why V 2 O 5 is a catalyst.
[1]
ii) Explain why V 2 O 5 can act as a catalyst in this reaction.
[1]
Assess your score
View Answer
c)
Excess ammonia is added to a solution of Cu2+ ions resulting in the substitution of 4 ligands.
Using section 15 of the data booklet, explain why this reaction results in a shift in the wavelength of light absorbed by the Cu2+ complex.
[1]
Assess your score
View Answer
Previous Question Next Question
Assess your score
View Answer
Assess your score
View Answer
Assess your score
View Answer
Previous Question Next Question
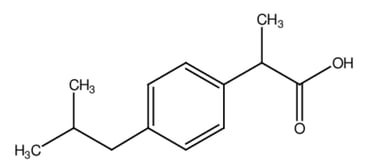
a)
Ibuprofen is a common non-steroidal anti-inflammatory drug (NSAID). It contains a benzene ring and a carboxylic acid at the end of one of the branches.
Deduce the number of resonance structures possible in the deprotonated form of ibuprofen.
[1]
Assess your score
View Answer
b)
Deduce the number of:
i)
Sigma (σ) bonds in ibuprofen
[1]
ii)
Pi (π) electrons in ibuprofen
[1]
Assess your score
View Answer
c)
The ibuprofen molecule contains both sp3 and sp2 hybridised orbitals.
i)
Identify how many sp3 hybrid orbitals are present.
[1]
ii)
Identify how many sp2 hybrid orbitals are present.
[1]
Assess your score
View Answer
d)
Explain why the benzene ring is a regular, planar hexagon.
[3]
Assess your score
View Answer
Previous Question Next Question
a)
State the formula for calculating the standard enthalpy change of reaction, ΔH r
Assess your score
View Answer
b)
Use section 11 of the data booklet to calculate the enthalpy change, in kJ mol-1 , for the following reaction.
Cl2 + H2 → 2HCl
Assess your score
View Answer
c)
State whether the energy change for the reaction in part (b) is endothermic or exothermic.
Assess your score
View Answer
d)
Using section 11 of the data booklet, calculate the enthalpy change of reaction, ΔHr , in kJ mol-1 for the following reaction.
CH4 + Cl2 → CH3 Cl + HCl
Assess your score
View Answer
Previous Question Next Question
a)
A student measured the energy change when 1.35 g of zinc was added to 50 cm
3 of 0.5 mol dm
-3 copper sulfate, CuSO
4 (aq), solution. The initial temperature of 21
o C was recorded before the addition of the zinc and a temperature reading was taken every 30 seconds.
Use the graph to determine the overall temperature change for the reaction
Assess your score
View Answer
b)
Calculate the enthalpy change for the reaction in kJ mol-1 .
Assess your score
View Answer
c)
Calculate the percentage error between your value for the enthalpy change of reaction and the literature value of -217 kJ mol-1 . Give your answer to two significant figures.
Assess your score
View Answer
d)
Explain why your calculated value for the enthalpy change of reaction is different from the literature value of -271 kJ mol-1 .
Assess your score
View Answer
Previous Question Next Question
a)
Define the term nucleophile .
[2]
Assess your score
View Answer
b)
Explain why the hydroxide ion, OH- , is a stronger nucleophile than water.
[2]
Assess your score
View Answer
c)
State the two ways a nucleophilic substitution reaction can occur.
[1]
Assess your score
View Answer
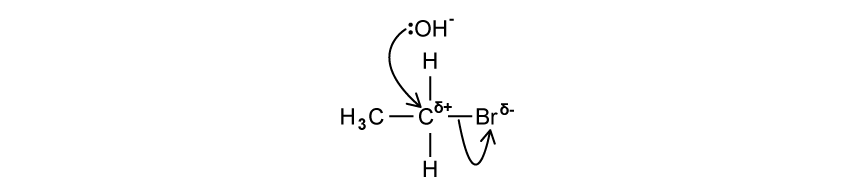
d)
State the the name of the mechanism occurring in the image below which will form ethanol in one step.
[1]
Assess your score
View Answer
Previous Question Next Question
a)
Ethane-1,2-diol, C2 H6 O2 , can be distinguished from ethanedioic acid,C2 H2 O4 , by a number of analytic techniques including MS, IR and NMR
The MS of these molecules is shown below.
Which spectrum belongs to each molecule? Justify your answer.
Spectrum A
Spectrum B
Assess your score
View Answer
b)
The IR spectra of ethane-1,2-diol, C2 H6 O2 , and ethanedioic acid dihydrate,C2 H2 O4 .2H2 O, are shown in spectrum C and D . Use Section 26 of the Data Booklet to answer this question.
Spectrum C D
Which spectrum belongs to each molecule? Justify your answer.
Assess your score
View Answer
c)
The 1 H NMR spectrum of ethane-1,2-diol is shown in spectrum E . Explain the significance of the spectrum.
Spectrum E
Assess your score
View Answer
Previous Question Next Question
Assess your score
View Answer
Assess your score
View Answer
Assess your score
View Answer
d)
Suggest two modifications to the procedure which would make the result more reliable.
Assess your score
View Answer
Previous Question











 Spectrum D
Spectrum D