 This section covers the foundations of atomic structure and explains the numerical values given on the periodic table. It is a relatively straightforward part of the course in terms of concepts, but a firm grip of the specific terms and the calculations are needed before you can move on to the challenges to come.
This section covers the foundations of atomic structure and explains the numerical values given on the periodic table. It is a relatively straightforward part of the course in terms of concepts, but a firm grip of the specific terms and the calculations are needed before you can move on to the challenges to come.
Ensure you are confident using the terms below and learn the asterisked* definitions
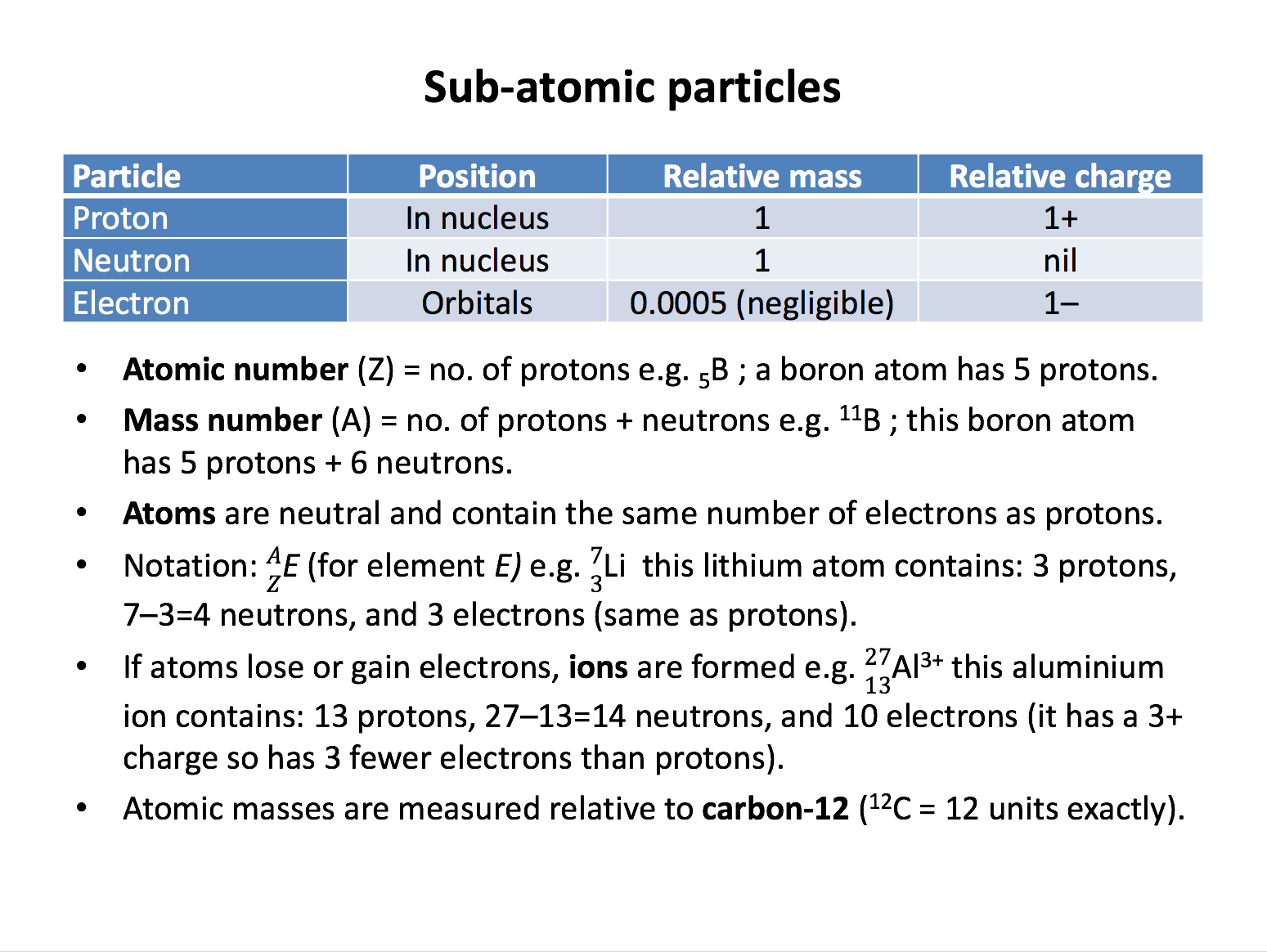
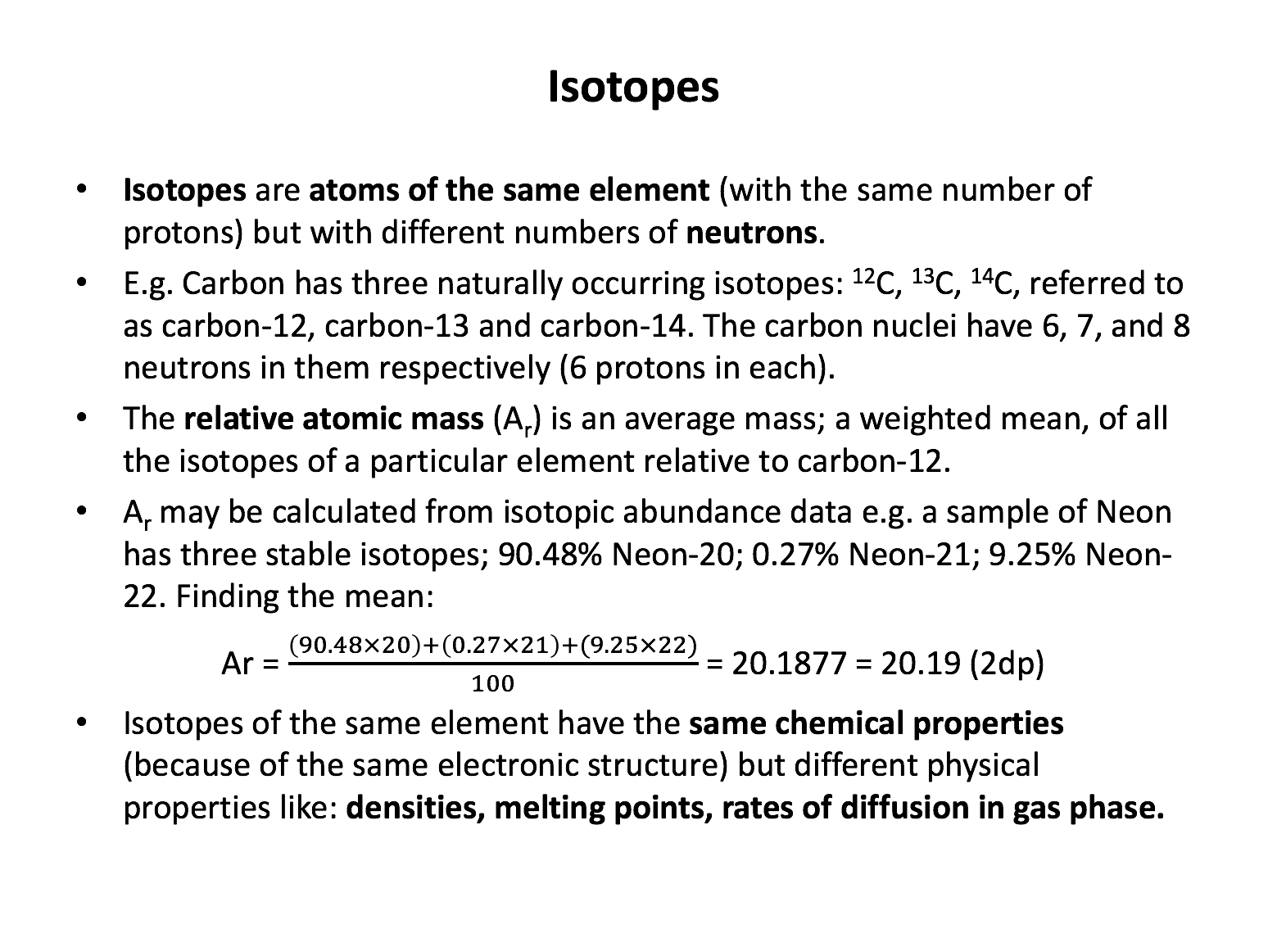
proton, neutron, electron, atomic number, mass number, relative atomic mass*, isotopes*
The relative atomic mass is measured relative to which elemental isotope?
All atomic masses are measured relative to Carbon-12 having a mass of exactly 12 units.
Which is the correct description of the position of the sub-atomic particles in an atom?
The nucleus contains the protons (positive) and neutrons (neutral) packed together. The electrons (negative) are found outside the nucleus in energy levels, or shells.
Which is the correct number of protons, neutrons and electrons in the magnesium-25 atom?
The number given after the dash in this format refers to the mass number, which is equal to protons plus neutrons. The number of protons is given by the atomic number found on the periodic table. The atomic number of magnesium is 12. The number of electrons is equal to the number of protons in a neutral atom. So magnesium-25 has 12 protons, (25 minus 12 = ) 13 neutrons and 12 (same number as protons) electrons.
Which is the correct number of protons, neutrons and electrons in the sodium-23 ion (Na+)?
The number given after the dash in this format refers to the mass number, which is equal to protons plus neutrons. The number of protons is given by the atomic number found on the periodic table. The atomic number of sodium is 11. The number of electrons is equal to the number of protons in a neutral atom, but this is 1+ positive ion, so it has one fewer electrons than protons. So the sodium-23 ion (Na+) has 11 protons, (23 minus 11 = ) 12 neutrons and 10 (one fewer than protons) electrons.
Which is the correct number of protons, neutrons and electrons in the chemical species \(\begin{smallmatrix} 27\\ 13 \end{smallmatrix}\)Al3+?
The upper number is the mass number, which is equal to protons plus neutrons. The lower number is the atomic number which is the number of protons. The number of electrons is equal to the number of protons in a neutral atom, but this is 3+ positive ion, so it has three fewer electrons than protons. So the species has 13 protons, (27 minus 13 = ) 14 neutrons and 10 (three fewer than protons) electrons.
Which is the correct number of protons, neutrons and electrons in the chemical species \(\begin{smallmatrix} 18\\ 8 \end{smallmatrix}\)O2−?
The upper number is the mass number, which is equal to protons plus neutrons. The lower number is the atomic number which is the number of protons. The number of electrons is equal to the number of protons in a neutral atom, but this is 2− negative ion, so it has two more electrons than protons. So the species has 8 protons, (18 minus 8 = ) 10 neutrons and 10 (two more than protons) electrons.
A sample of an element Z contains two isotopes, 80% 82Z and 20% 84Z. What is the relative atomic mass of Z in this sample?
The relative atomic mass is found by finding the mean (average) mass. Assume that the percentage given is the number of atoms (100 atoms in total) and calculate a mean; 80 atoms at mass 82, and 20 atoms at mass 84, divided by 100 atoms in total:
\(\frac{(80×82)+(20×84)}{100}\)= 82.4
A sample of element Y has a relative atomic mass of 39.1. Which could be the correct distribution of element Y's isotopes?
Without a calculator (for trial and error): The separation betweeen the two isoptpes (39 and 41) is 2 units. The average mass lies .1 of a unit close to 39 and 1.9 of a unit close to 41. That is 1/20th close to 39 and 19/20ths close to 41. The distribution is therefore 95% to 5% in favour of 39Y.
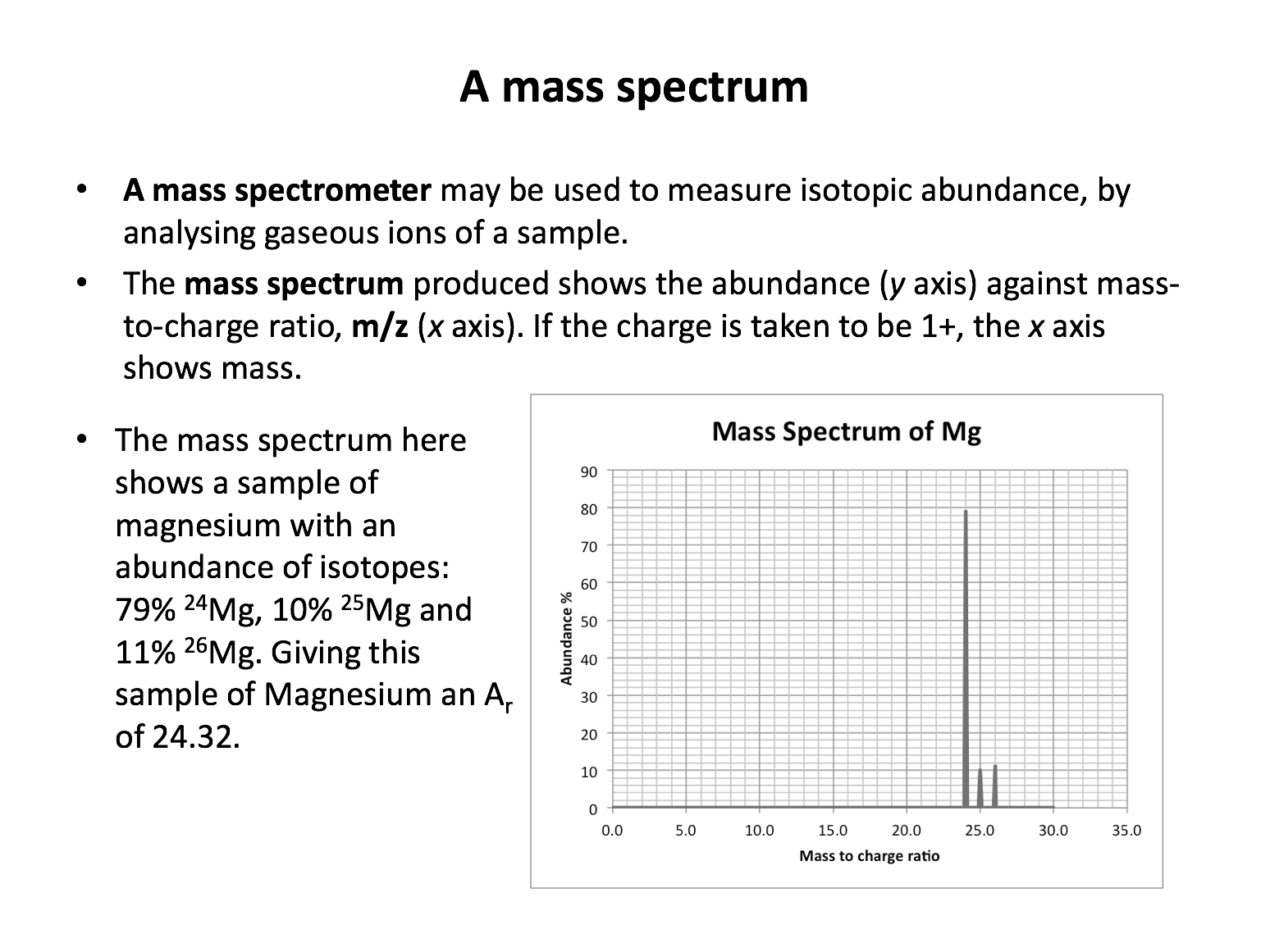
Look at the mass spectrum of X below:
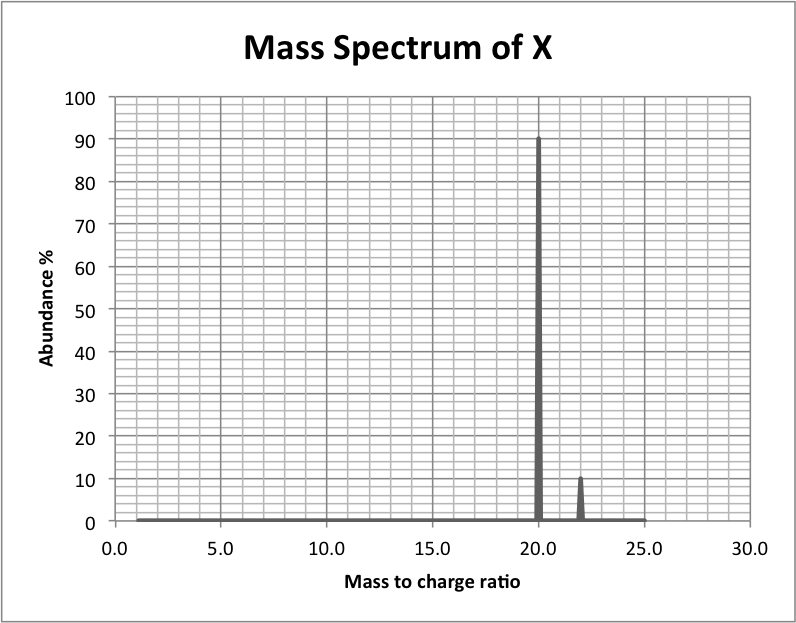
What is the relative atomic mass of X?
The spectrum shows 90% 20.0 and 10% 22.0. Without a calculator: The separation betweeen the two isoptpes (20 and 22) is 2 units. The average mass lies 90% closer to 20 than to 22. 90% of 2 units is 1.8 units. So the mass lies 1.8 units closer to 20 than to 22; thus 20.2 is the correct answer.
Paper 1
Core (SL&HL): Atomic Structure core (SL and HL) paper 1 questions
AHL (HL only): Atomic Structure AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Atomic Structure core (SL & HL) paper 2 questions
AHL (HL only): Atomic Structure AHL (HL only) paper 2 questions
How much of The nuclear atom have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn