Topic 12.1
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which element is in group 13?
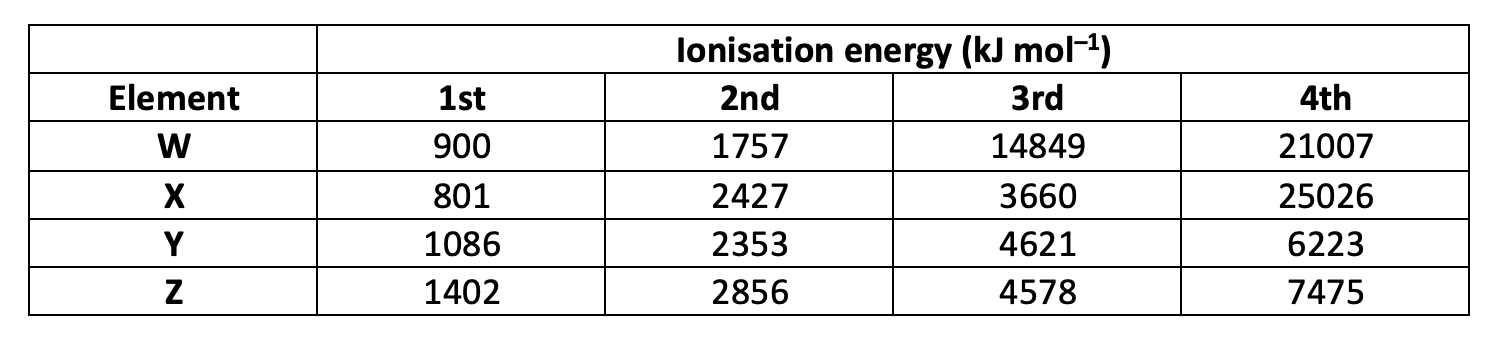
Group 13 elements (like B or Al) have the largest jump in ionisation energies (IEs) between the removal of the third and fourth electrons, because the first three electrons are removed from a p sub-level and s sub-level that are all within the same energy level (The electron configuration of Boron for example is 1s2 2s2 2p1). The fourth electron, however, is removed from a new energy level which is lower in energy, closer to the nucleus with stronger nuclear attraction and less shielding making the electrons more difficult to remove.
Element X has gradually increasing 1st to 3rd IEs and then a dramatic jump (much greater in comparison to the differences between the first three IEs) in IE to the 4th, so element X is most likely to be in group 13.
X is the correct answer.
Which graph shows the trend in first ionisation energies of elements across period 2?
The graph showing the trend in first ionisation energies (1st IE) across the periodic table needs to be learned (at least up to calcium, atomic number 20) such that you are able to sketch the graph. The graph is shown below:
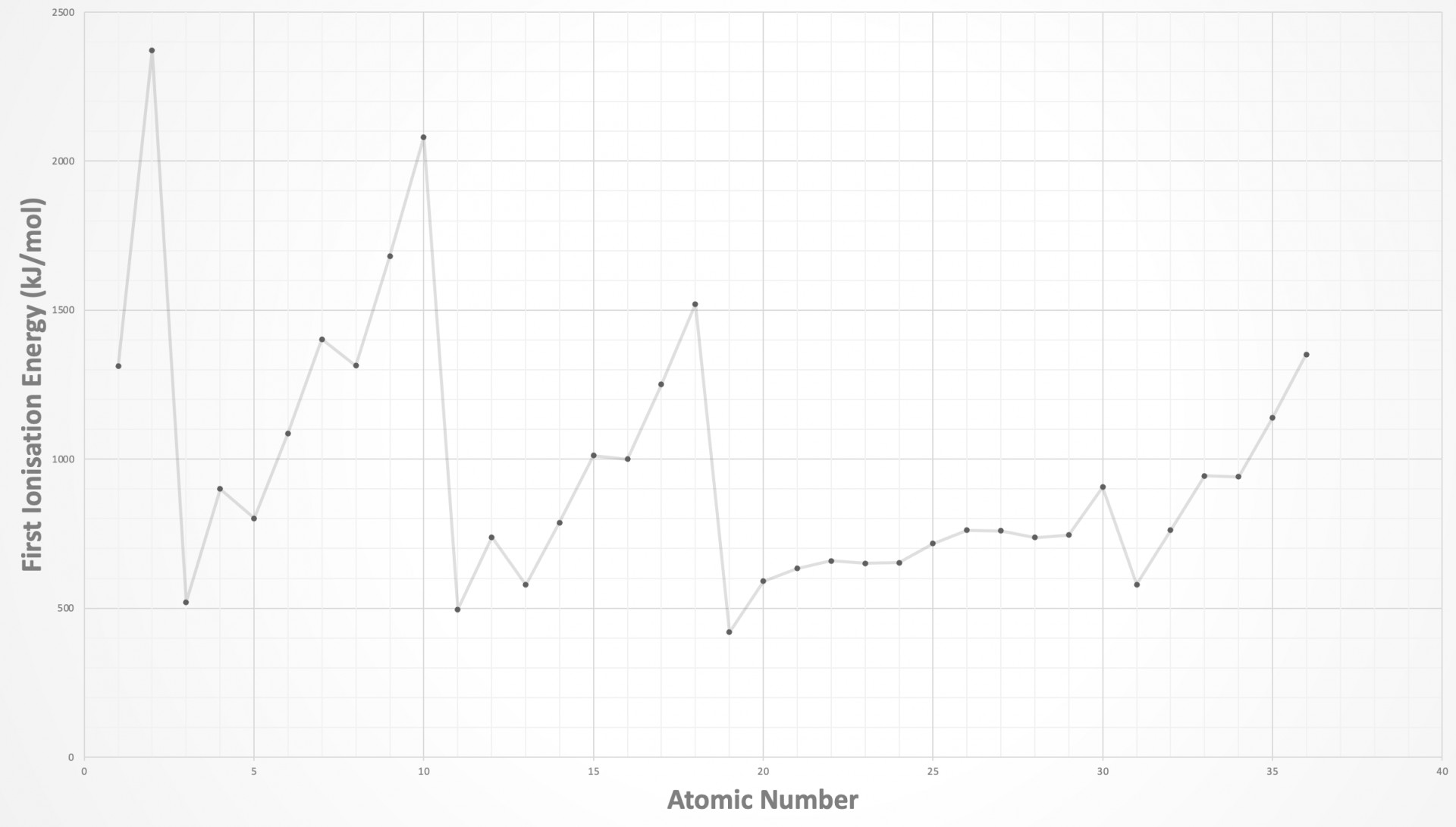
There are only two elements in the first period, H and He.
The best way to 'anchor' the position of a graph showing a section of the trend in ionisation energies is to look for the highest points which are always in group 18 (Noble gases) and the lowest points which are always group 1 (Alkali metals). Also, notice the similar pattern across the eight elements in period 2 and period 3. There are regular dips in 1st IE in the pattern 2, 3, 3 across both periods, before a big drop to the next period. The pattern is explained in the video: First ionisation energies
The 2, 3, 3 pattern is therefore seen across period 2 in the correct answer:
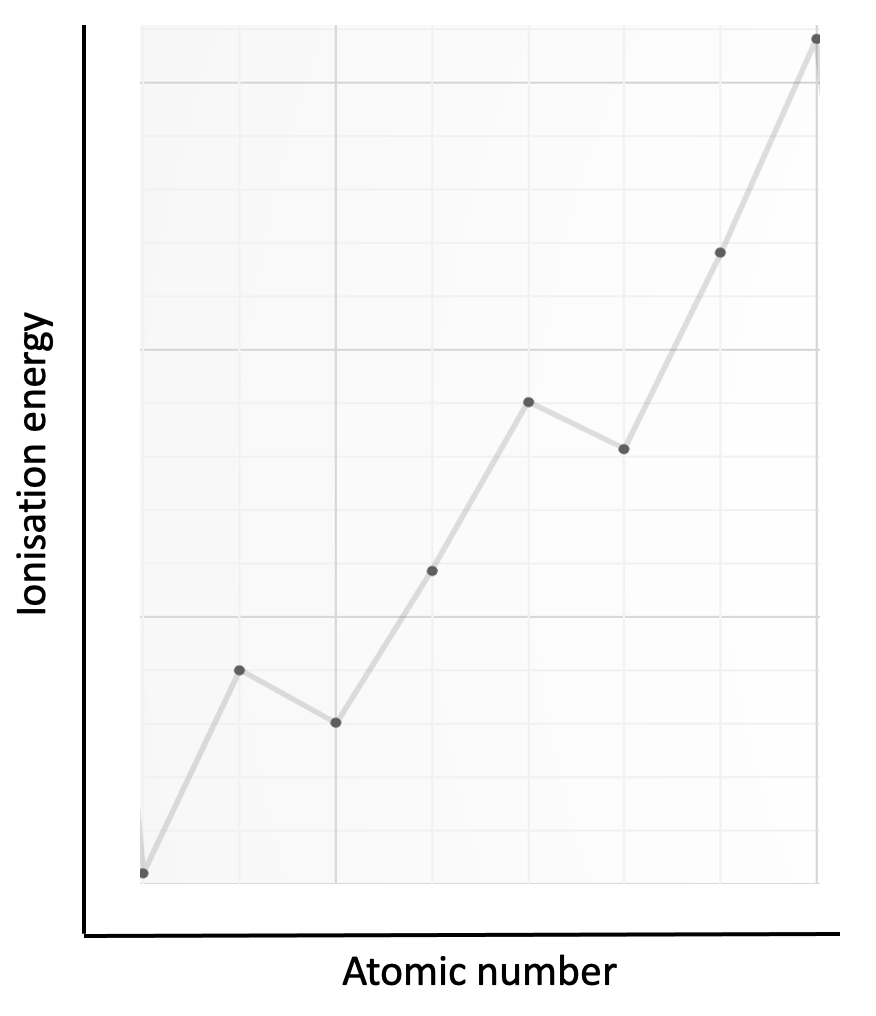
Which element is represented by the first eight successive ionisation energies shown on the graph?
The electron configurations for the four possible elements are; aluminium 1s22s22p63s23p1 ; silicon 1s2 2s2 2p6 3s2 3p2 ; phosphorus 1s2 2s2 2p6 3s2 3p3 and argon 1s22s22p63s23p6.
The graph has the largest jump in ionisation energy (IE) between the 5th and 6th electron and a relatively smaller jump between the 3rd and 4th electron. The large jump in IE (5th to 6th) will correspond to a new energy level, and the smaller jump to a new sub-level.
Phosphorus, P, is the only element in the list with a change in energy level after the removal of five electrons (3p3 and 3s2 removed) and it also has a change in sub-level (3p to 3s) after three electrons have been removed.
P is therefore the correct answer.
The first four successive ionisation energies for two elements, X and Z, are given below (in kJ mol−1).
Which pair of elements represent X and Z?.
X: 1314, 3388, 5301, 7469
Z: 787, 1577, 3232, 4356
The electron configurations for the four possible elements are; oxygen 1s2 2s2 2p4 ; silicon 1s2 2s2 2p6 3s2 3p2 ; carbon 1s2 2s2 2p2 and sulfur 1s22s22p63s23p4.
Element X has a similar difference of approx. 2000 units between each ionisation energy (IE), with no larger jumps in IE. For oxygen there will be no larger jumps in ionisation energy, since the first four electrons are all removed from the 2p sub-level, so element X is oxygen. (Carbon would show a slightly larger jump is IE between the second and third IE; 2p to 2s.)
Element Z has the largest difference of approx. 1700 units between the second and third IEs. For silicon the largest jump will be between the second and third IEs (3p to 3s), so element Z is silicon. (Sulfur would have a similar difference between all of the first four IEs as all electrons are removed from 3p.)
The correct answer is therefore X is oxygen; Z is silicon.
Which transition on the diagram could represent the first ionisation energy of hydrogen?
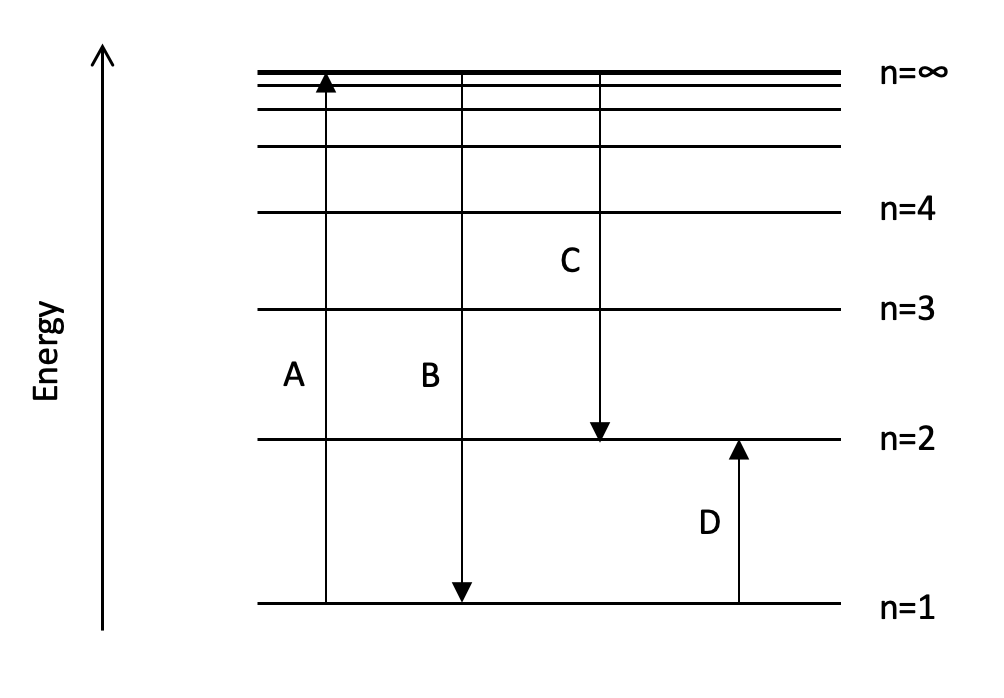
A hydrogen atom has only one electron. In the ground state, the electron is in the 1s sub-level (in the n=1 energy level). The first ionisation energy involves removing an electron from every atom in one mole of gaseous atoms, this can be represented as moving the ground state electron to the n=∞ energy level.
Therefore an electron transition from n=1 to n=∞ could represent the first ionisation energy of hydrogen.
A is therefore the correct answer.
The graph shows the first ionisation energies of some consecutive elements in order of atomic number. Which statement is correct?
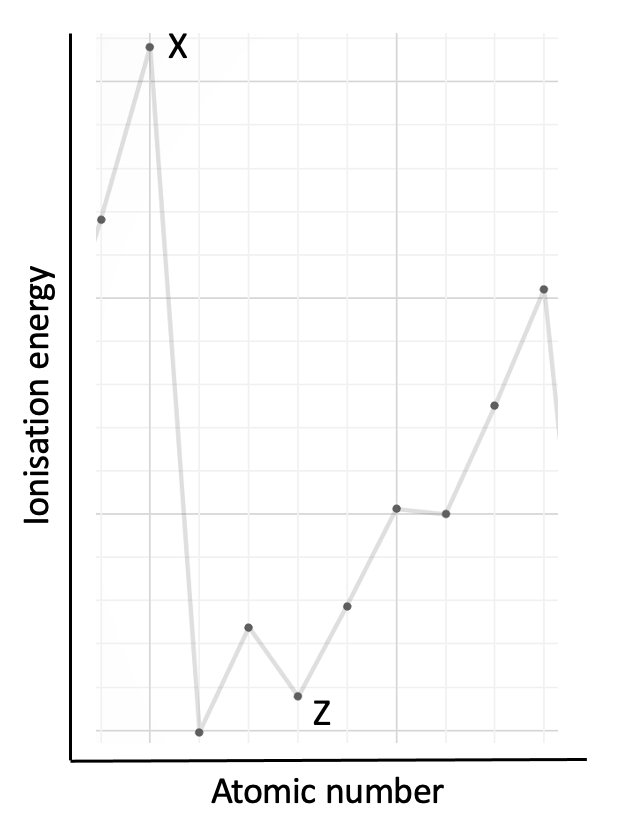
This graph of ionisation energy does not begin with hydrogen. The best way to 'anchor' the position of a graph showing a section of the trend in ionisation energies is to look for the highest points which are always in group 18 (Noble gases) and the lowest points which are always group 1 (Alkali metals). Look also for the 2, 3, 3 pattern in elements that is seen across period 2 and period 3. See the graph below:

The graph showing the trend in first ionisation energies (1st IE) across the periodic table needs to be learned (at least up to calcium, atomic number 20) such that you are able to sketch the graph. The pattern is explained in the video: First ionisation energies
In this question element X is at a high point on the graph that corresponds to group 18. Element Z is in group 13.
The correct answer is therefore Element X is in group 18.
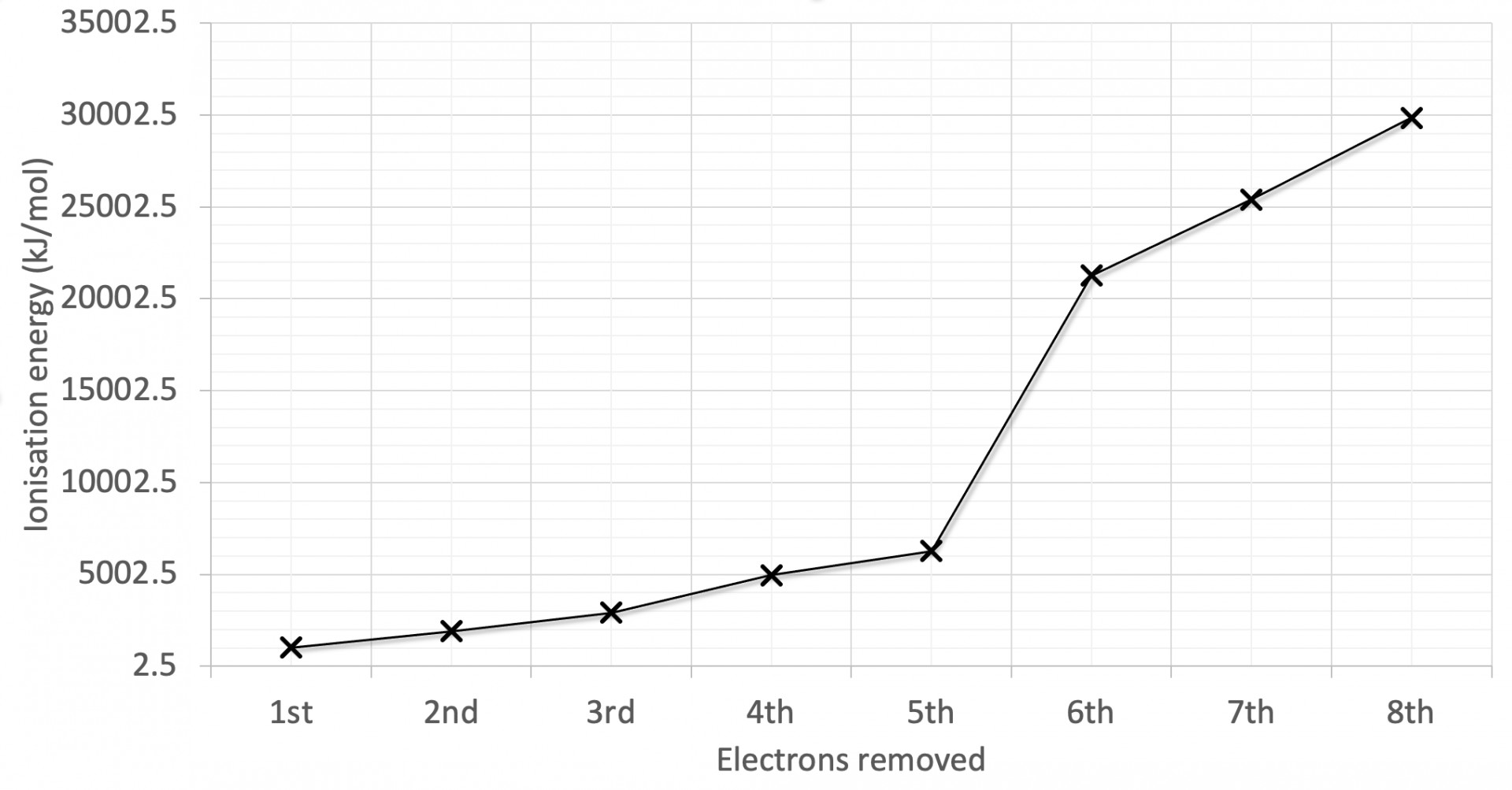
The first eight successive ionisation energies for an element are shown on the graph. Why is there a relatively large increase in successive ionisation energy between the 4th and 5th electrons removed?
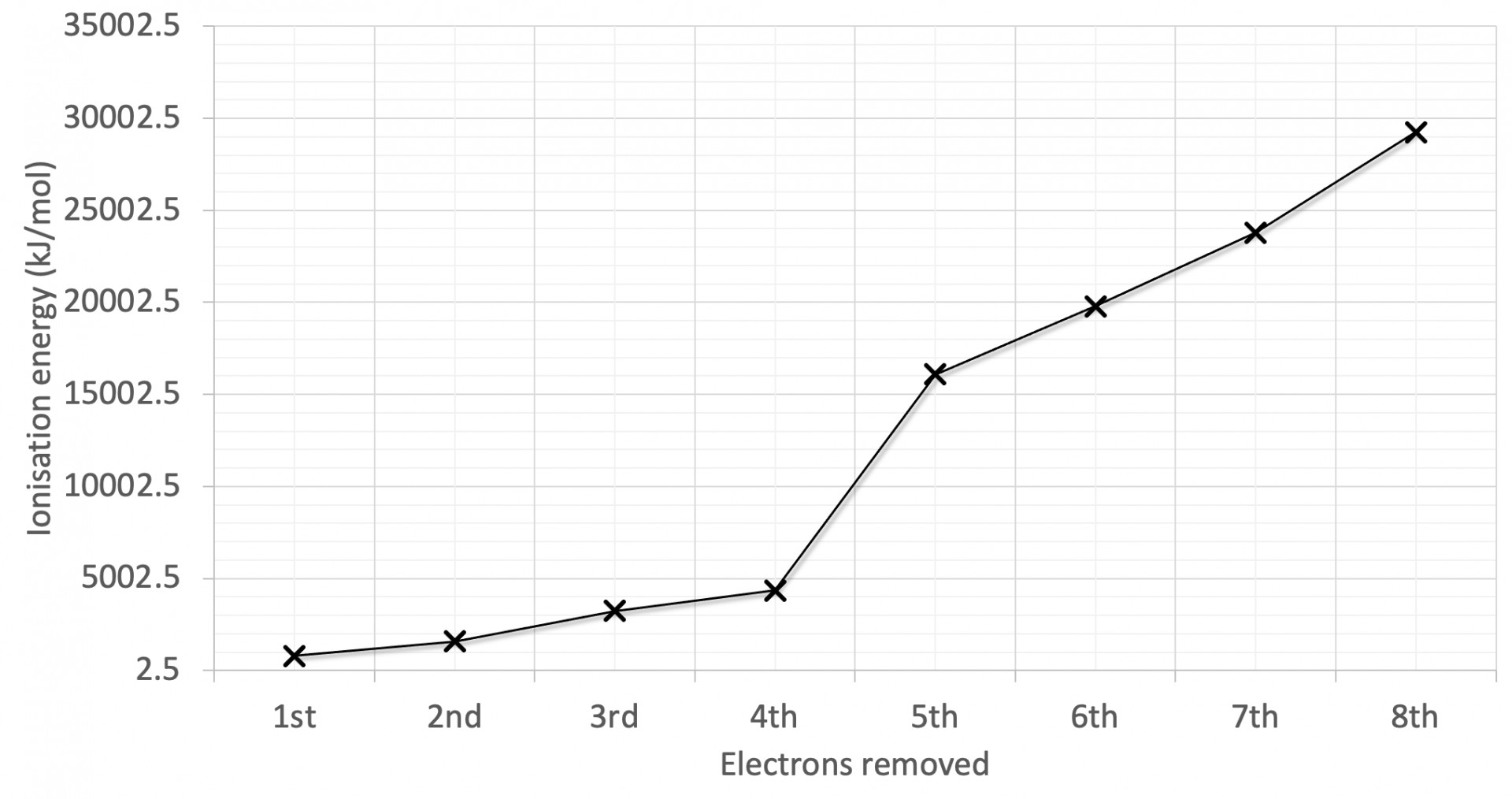
The graph has the largest jump in ionisation energy (IE) between the 4th and 5th electrons and a relatively smaller jump between the 2nd and 3rd electrons.
Large jumps in successive IEs will always correspond to new (lower) energy levels, and the smaller jumps will often correspond to a new (lower) sub-levels.
The 5th electron removed is in a lower energy level is therefore the correct answer.
The graph shows the first ionisation energies of the first twenty consecutive elements in order of atomic number. Which statement correctly explains a decrease in ionisation energy?
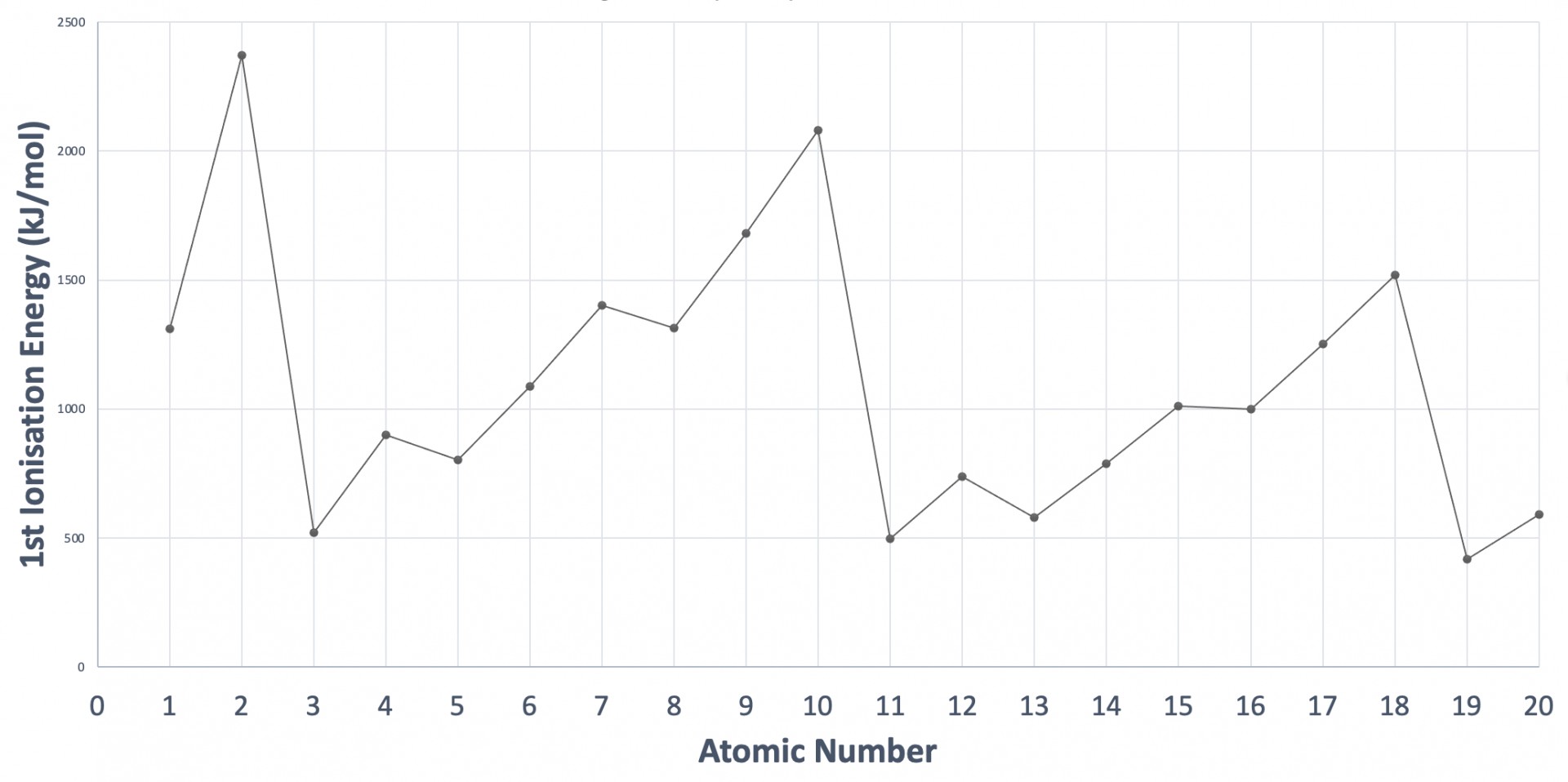
The graph showing the trend in first ionisation energies (1st IE) across the periodic table needs to be learned (at least up to calcium, atomic number 20) such that you are able to sketch the graph. The pattern is explained in the video: First ionisation energies
The large decreases across the graph are due to an electron being removed from a new energy level of higher energy e.g. element 2 is Helium (1s2) to element 3, which is Lithium (1s2 2s1).
The smaller decreases between elements 4 to 5 and 12 to 13 are due to an electron being removed from a new sub-level of higher energy e.g. element 4 is Berylium (1s22s2) to element 5, which is Boron (1s2 2s2 2p1).
The decreases in IE between elements 7 to 8 (N to O), and 15 to 16 (P to S) are due to the electron being removed from a p orbital in which the electrons have been required to pair-up, creating additional electron-electron repulsion (relative to the previous three elements in which the electron removed occupies the p orbital singularly).
The correct answer is therefore Element 16 has greater electron-electron repulsion than element 15 due to electron pairing.
A period 2 element, X, reacts with oxygen to form an ionic oxide with empirical formula X2O.
Which correctly represents the first three successive ionisation energies (in kJ mol−1) for element X?
The element X forms an ionic compound with oxygen, so must be a metal (ionic compounds are always metal and non-metal) and has formula X2O, so X forms a 1+ ion. X is in period 2, so it must be Lithium.
Lithium has electron configuration 1s2 2s1, so there will be a large relative increase in ionisation energy (IE) between the 1st and 2nd IEs, as the second electron is removed from a new energy level.
The only series that shows a large relative increase in IE between 1st and 2nd IE is 520, 7298, 11815. Therefore, this is the correct answer.
The first ionisation energies (in kJ mol−1) of five consecutive elements on the periodic table are:
1251, 1521, 419, 590, 631.
What could these elements be?
Large decreases in first ionisation energy (like 1521 to 419) across the periodic table always indicate a new period. The graph showing the trend in first ionisation energies (1st IE) across the periodic table needs to be learned (at least up to calcium, atomic number 20) such that you are able to sketch the graph. The pattern is explained in the video: First ionisation energies

In this question the 1st IEs 1251 and 1521 suggest the last two elements in a period, as there is a big drop to 419 suggesting a new period.
The correct answer is therefore The last two elements of one period and the first three elements of the next period.
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which of the following best represents the shape of an s-atomic orbital?
Orbitals are plots of electron position (90% probability) around the nucleus. They are three dimensional (not planar). An s-orbital is a sphere; a p-orbital is a three dimensional figure-of-eight.
So the answer here that best decribes as s-orbital is  .
.
The red figure-of-eight image represents a p-orbital.
For HL students only: The elongated blue image represents a sigma molecular orbital (two orbitals overlapping end on) and the elongated red image represents a pi molecular orbital (two p-orbitals overlapping side on).
What is the correct electronic configuration for an atom of copper?
The sub-levels of an atom are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s. Remember that 's' sub-levels hold a maximum of 2 electrons, 'p' hold 6, and 'd' hold 10.
A copper atom has 29 electrons in total (atomic number is 29), but the configuration is not what would be expected as the electrons in the 4s and 3d energy levels are 4s1 3d10 instead of filling the sub-levels in the conventional manner (4s2 3d9). Chromium and copper have unusual configurations and need to be learned.
Remember that [Ar] is shorthand and indicates the electron configuration of argon: 1s22s22p63s23p6.
The correct answer is therefore 1s2 2s22p63s23p64s13d10
Which is the correct number of protons, neutrons and electrons in the calcium ion \(\begin{smallmatrix} 44\\ 20 \end{smallmatrix}\)Ca2+?
The upper number is the mass number, which is equal to protons plus neutrons. The lower number is the atomic number which is the number of protons. The number of electrons is equal to the number of protons in a neutral atom, but this is 2+ positive ion, so it has two fewer electrons than protons.
So the species has 20 protons, (44−20) 24 neutrons and 18 electrons (two fewer than protons).
Which statements are correct with regard to the absorption spectrum of hydrogen?
1: The lines are produced when electrons move from higher to lower energy levels
2: The lines converge at lower wavelengths
3: The lines in the visible region of the electromagnetic spectrum are seen as coloured lines
The line absorption spectrum of hydrogen is composed of discrete lines that represent particular frequencies. Lines are produced by electron transitions from lower to higher energy levels (for absorption; from higher to lower for emission).
The lines converge at higher frequencies/energies which corresponds to lower wavelengths.
Any lines in the absorption spectrum are not seen as coloured lines, but as black lines (an absence of colour) as electromagnetic radiation is absorbed (not emitted).
Thus the answer is 2 only.
Which statement about 65Zn2+ and 65Cu+ is correct?
The upper number on the left is the mass number, which is equal to protons plus neutrons. The atomic number for zinc is 30 and for copper is 29; this is the number of protons, shown on the periodic table.
The number of electrons is equal to the number of protons in a neutral atom, but these species are positive ions so will have more protons than electrons.
65Zn2+ therefore has 30 protons, 65−30=35 neutrons, and 30−2=28 electrons (as it is a 2+ ion).
65Cu+ therefore has 29 protons, 65−29=36 neutrons, and 29−1=28 electrons (as it is a 1+ ion).
The correct answer is therefore Both have the same number of electrons.
In which set do all the species contain more neutrons than electrons?
The upper (superscript) number on the left is the mass number, which is equal to protons plus neutrons. The atomic number, which is the number of protons is shown on the periodic table. The number of electrons is equal to the number of protons in a neutral atom but negative ions will have correspondingly more electrons and positive ions correspondingly fewer.
8Li+ has 8−3=5 neutrons and 3−1=2 electrons
8Li has 8−3=5 neutrons and 3 electrons
31P3− has 31−15=16 neutrons and 15+3=18 electrons
31P has 31−15=16 neutrons and 15 electrons
15N3− has 15−7=8 neutrons and 7+3=10 electrons
15N has 15−7=8 neutrons and 7 electrons
The correct answer is therefore 8Li+, 31P, 15N as this is the only set in which all species contain more neutrons than electrons.
Which electron transition in the hydrogen emission spectrum emits radiation with the longest wavelength?
Longer wavelength corresponds to lower frequency/lower energy. So the longest wavelength will correspond to the electron transition of lowest energy.
The question also asks about emission - energy given out - so the transition must be from a higher to lower energy level.
The energy gaps between the levels get smaller at higher energy.
Therefore the smallest energy transition that is also an emission is n=6 to n=5, which is therefore the correct answer.
The full electron configuration of an atom of an element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3.
To which group and period does the element belong?
The electron configuration reflects an element's position on the periodic table.
The easiest way to solve this is to count the total number of electrons (23); as this is an atom, the number of electrons will be equal to the number of protons, so the element is vanadium.
Vanadium is in Group 5, Period 4.
Fluorine has only one stable isotope. Which statement is incorrect for the fluoride ion \(\begin{smallmatrix} 19\\ 9 \end{smallmatrix}\)F−?
The upper number is the mass number, which is equal to protons plus neutrons (19 sub-atomic particles in the nucleus). The lower number is the atomic number which is the number of protons. The number of electrons is equal to the number of protons in a neutral atom, but this is a 1− negative ion, so it has one more electron than protons.
So this fluoride ion has 9 protons, (19−9=)10 neutrons and 10 electrons (1s2 2s2 2p6).
The question states that there is only one stable isotope of fluorine so all stable fluoride ions will have 10 neutrons.
The correct answer is therefore The ion has the same electron configuration as an atom of argon as this is incorrect: Argon has an electron configuration of 1s2 2s2 2p6 3s2 3p6.
What is the number of protons and the number of neutrons in 82Kr?
The upper number on the left is the mass number, which is equal to protons plus neutrons. The atomic number for krypton is 36, which is the number of protons; shown on the periodic table.
82Kr therefore has 36 protons and (82−36=)46 neutrons.
The correct answer is therefore 36 protons, 46 neutrons.
What is the correct condensed electronic configuration for the V3+ ion?
The sub-levels of an atom are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s.
Remember that 's' sub-levels hold a maximum of 2 electrons, 'p' hold 6, and 'd' hold 10, and that [Ar] is shorthand and indicates the electron configuration of argon: 1s22s22p63s23p6.
A vanadium atom has 23 electrons in total (atomic number is 23) so the condensed atomic electron configuration is [Ar] 4s2 3d3.
The V3+ ion has a 3+ charge so has lost three electrons. These electrons are lost from the 4s orbital before the 3d. Remember that '4s are gained first and lost first'! So two electrons are lost from 4s and one electron is lost from 3d.
The correct answer is therefore [Ar] 4s0 3d2.
What is the maximum number of electrons that can occupy the n=3 energy level?
Each and every orbital can hold two electrons. Any s sub-level contains one orbital and can therefore hold two electrons. Any p sub-level contains three orbitals and can therefore hold six electrons. Any d sub-level contains five orbitals and can therefore hold ten electrons. Any f sub-level contains seven orbitals and can therefore hold fourteen electrons.
The n=3 energy level consists of the 3s, 3p, and 3d sub-levels.
Thus the answer is 18 (2+6+10).
How much of Atomic Structure AHL (HL only) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn