Topics 11.1 to 11.3
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
What is the index of hydrogen deficiency for aspirin?
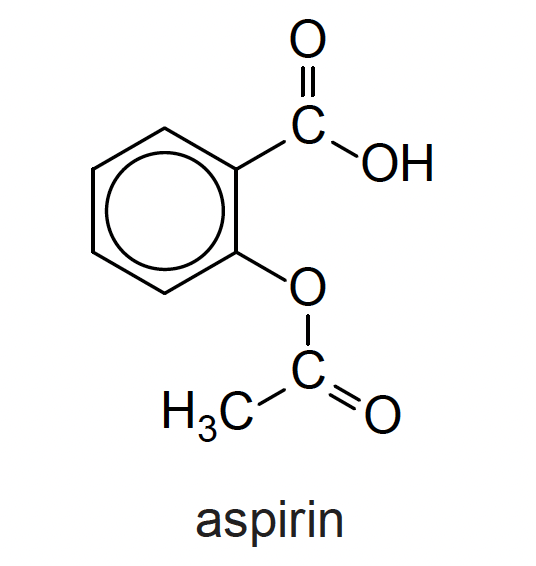
The index of hydrogen deficiency is a measure of the 'double bond equivalents' in a molecule.
Any ring counts as 1, as does a double bond. So a benzene ring counts as four, that is three double bonds and a ring!
Therefore the correct answer is 6.
That is five double bonds and one ring.
The equation can be used (but it is easier just to count the rings and double bonds) where the letters represent the appropriate elements and X represents any halogen (oxygen can be ignored): (Aspirin C9H8O4)
\(IHD = (C+1)-{(H-N+X) \over 2}\)
IHD = (9+1) – (8–0+0)/2 = 10 – 4 = 6
Which techniques would be effective in distinguishing between ethanol (CH3CH2OH) and ethanal (CH3CHO)?
1: Infrared (IR) spectroscopy
2: Mass spectrometry
3: 1H-NMR spectroscopy
1H-NMR spectroscopy would be effective because ethanol and ethanal have different numbers of hydrogens in different environments; (3:2:1) and (3:1) respectively.
Infrared (IR) spectroscopy would be effective because the O−H bond in ethanol would give a different band in the spectrum to the C=O bond in ethanal.
Mass spectrometry would be effective because ethanol and ethanal have different molecular masses (and different fragmentation patterns).
1, 2 and 3 is therefore the correct answer.
How should the difference between 33.0±0.3 and 8.0±0.2 be shown?
If we are processing an uncertainty by addition or subtraction we should add the absolute/raw uncertainties.
0.3+0.2=0.5
Therefore the correct answer is 25.0±0.5
Complete this question without a calculator: A student obtained the following data to calculate q, using q = mcΔT.
Mass (m) is 20.0±0.2g
Specific heat capacity (c) is 4.18 Jg–1K–1 (no uncertainty)
ΔT is 10±2 °C
Which is the percentage uncertainty (±%) in the final calculated value of q?
To process uncertainties the absolute uncertainties should be converted to % uncertainties and then added together:
(Percentage uncertainty = absolute uncertainty / measurement value × 100%)
0.2/20.0 × 100 is 1% and 2/10 × 100 is 20%
Thus the correct answer is 21 %.
Which region of the electromagnetic spectrum is used to identify bond vibrations in a molecule?
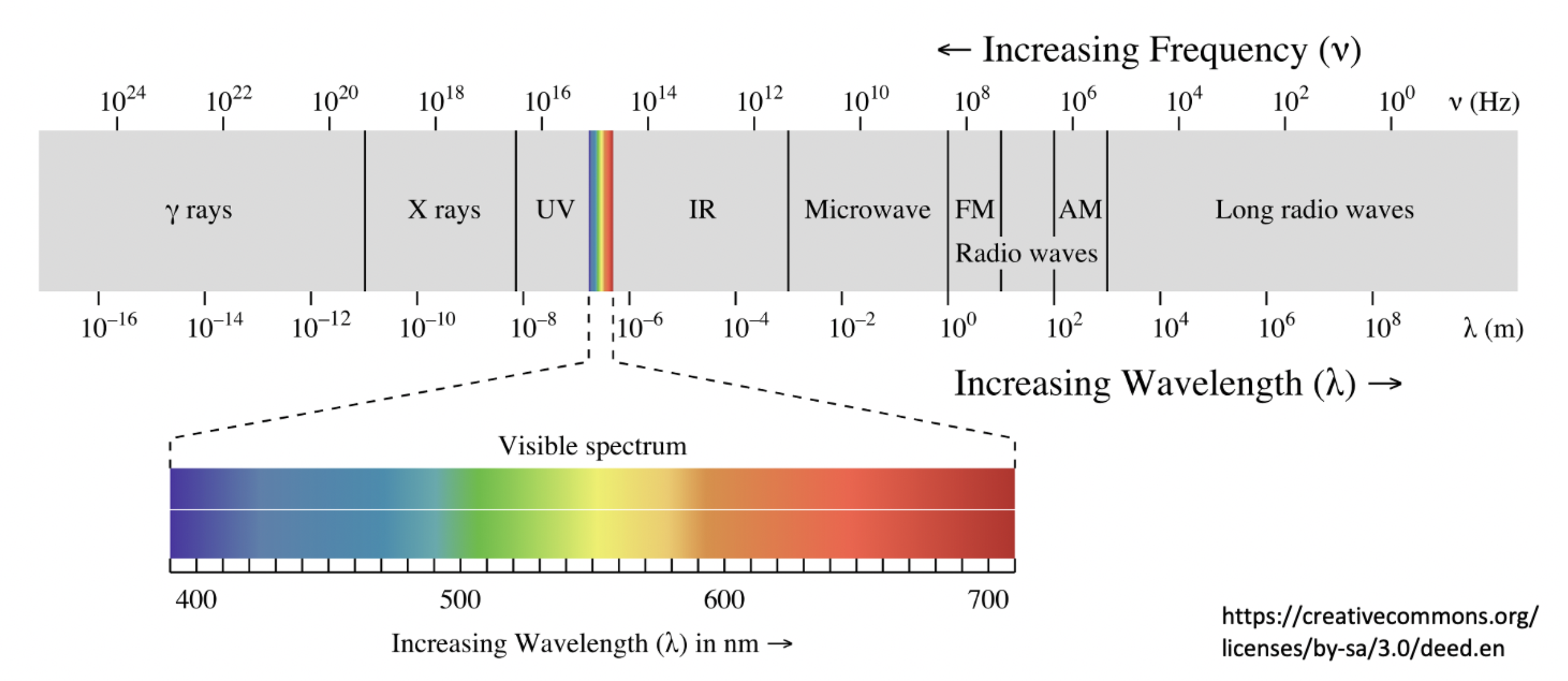
Bond vibrations can be identified by infrared (IR) spectroscopy.
Infrared (IR) is therefore the correct answer.
How should a measurement of 7.00g on a two decimal place balance be recorded?
When recording an uncertainty in chemistry, we tend to record the raw uncertainty to one significant figure, and to the smallest increment of the measurement.
Therefore the correct answer is 7.00±0.01
Which can 1H-NMR spectra always tell us about a molecule?
1H-NMR spectroscopy summary (Core):
Peaks: The number of peaks tells us the number of different hydrogen environments.
Ratio: The integration trace or whole number written adjacent to each peak tells us the ratio of the number of hydrogens in each environment.
Shift: The chemical shift table (data book) can help us with the types of hydrogens (functional groups etc.) in the molecule.
(Knowledge of spin-spin splitting is also required at HL)
Therefore The total number of hydrogen environments is the correct answer. Since 1H-NMR will not always tell us the total number of hydrogen atoms in a molecule (as sometimes we are just given the ratio).
(The bonds present in a molecule can be ascertained through IR spectroscopy, and molecular mass through mass spectrometry.)
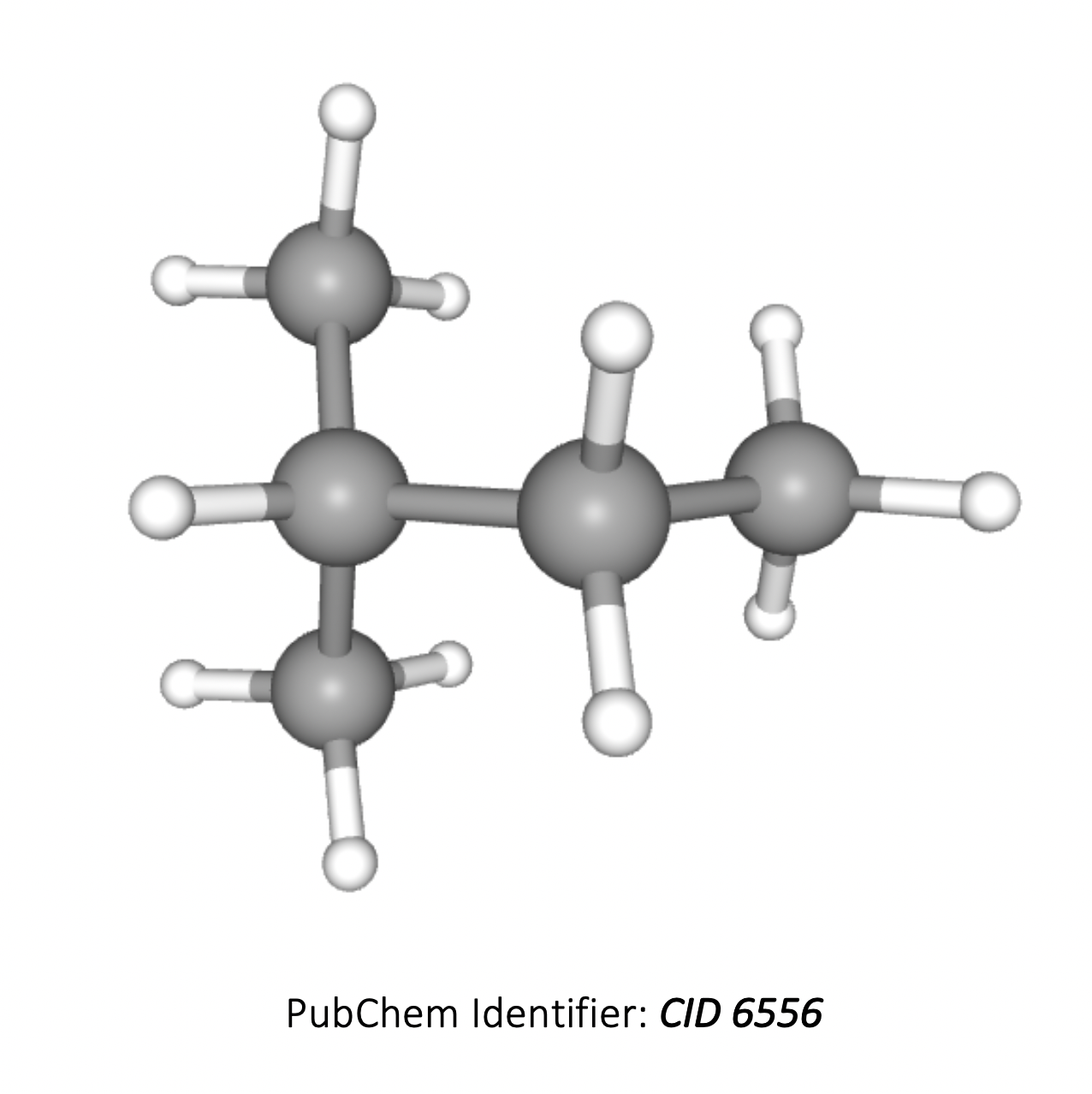
What is the ratio of areas under each signal in the 1H-NMR spectrum of 2-methylbutane?
The compound 2-methylbutane is (CH3)2CHCH2CH3 (3D image below). The hydrogens in the two CH3 groups to the left are equivalent, so form one environment. The hydrogens in the CH, CH2 and the CH3 group on the right all contain hydrogens which are in different environments, that is another three environments.
Therefore there are 4 different hydrogen environments in total, and the ratio of areas under each signal will be 6 : 1 : 2 : 3.
6 : 1 : 2 : 3 is therefore the correct answer.
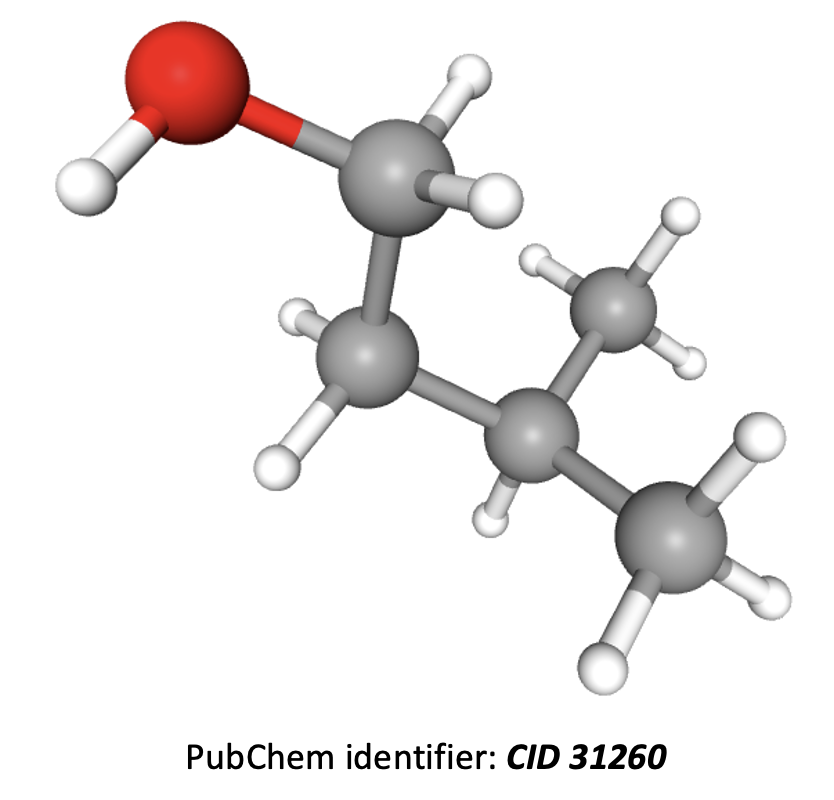
How many different hydrogen environments and therefore peaks will be present in an 1H-NMR spectrum of 3-methylbutan-1-ol?
(CH3)2CHCH2CH2OH
The compound 3-methylbutan-1-ol is shown in 3D below. The hydrogens in the two CH3 groups to the right are equivalent, so form one environment. The hydrogens in the CH, CH2, CH2, and OH groups, moving left across the image, all contain hydrogens which are in different environments; that is another four environments.
Therefore there are 5 different hydrogen environments in total.
5 is the correct answer.
A student records a volume reading from a burette to an uncertainty of ±0.05cm3. Which record of the reading is the most appropriate, given the uncertainty?
It is conventional in chemistry to quote raw uncertainties to the same precision (decimal places) as the reading, and to one decimal place.
Thus 12.25cm3 is the correct answer as it is the only answer given to two decimal places.
How much of Measurement core (SL and HL) paper 1 questions have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn