Topic 19.1
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
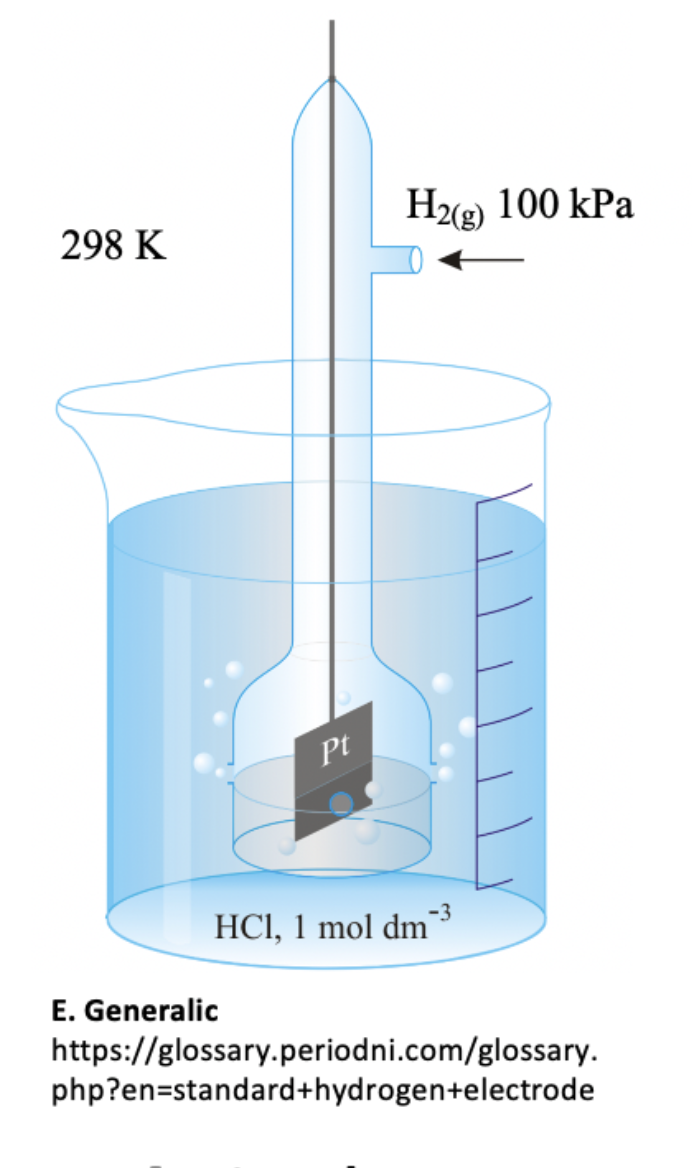
Which of the following species are components of the standard hydrogen electrode?
1: Pt (s)
2: H+ (aq)
3: OH− (aq)
Electrode potentials are measured against the standard hydrogen electrode.
The standard hydrogen electrode consists of a feed of hydrogen gas at 100 kPa onto an inert Pt electrode in 1 mol dm–3 hydrochloric acid all at 298 K.
The platinum electrode is coated with platinum black; finely powdered platinum to give a high surface area for the reaction to occur on:
H+(aq) + e– ⇌ ½H2(g) Eo = 0.00 V
Thus 1 and 2 only is the correct answer.
What are the products at the cathode (negative electrode) and anode (positive electrode) when concentrated KCl(aq) is electrolysed using inert electrodes?
The species discharged at the cathode/negative electrode (gaining e–) will be lower (less –ve) in the electrochemical series; water is lower than potassium so water is discharged, forming hydrogen gas (hydrogen is lower in the activity series than potassium). Without the data booklet we must remember that the least reactive metal/hydrogen is discharged.
The species discharged at the anode/positive electrode (losing e–) will be higher (less +ve) in the electrochemical series. Again, without the data booklet we must remember that the least reactive non-metal is discharged. Note! Exception: Chloride ions (½Cl2(g) + e– ⇌ Cl–(aq) Eo = +1.36 V) are preferentially discharged over water (½O2(g) + 2H+(aq) + 2e– ⇌ H2O(l) Eo = +1.23 V) at the positive electrode despite their Eo values (this is a kinetic effect that needs remembering).
Thus Cathode: H2 Anode: Cl2 is the correct answer.
Which factors affect the amount of product formed at the anode during the electrolysis of a molten metal halide?
1: Current
2: The charge on the metal ion
3: Time
During electrolysis of a molten metal halide, the metal ions will be discharged at the cathode (negative electrode) and the halide ions will be discharged at the anode (positive electrode).
The amount of charge passed can be found thus: charge (coulombs, C) = current (amps, A) × time (seconds, s), so both current and time will affect the amount of electrons and therefore the amount of product formed at the anode and cathode.
However, the metal ion is discharged at the cathode, rather than the anode, so the charge on the metal ion will have no impact on the amount of product at the anode.
Thus 1 and 3 only is the correct answer.
What is the cell potential for a voltaic cell consisting of the half-cells below?
Mn2+(aq) + 2e– ⇌ Mn(s) Eo = –1.18 V
Cu2+(aq) + 2e– ⇌ Cu(s) Eo = +0.34 V
In a voltaic cell the half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction).
The cell potential is the difference (as on a number line) in the potential of the two half-cells.
Or mathematically Eocell = Eocathode – Eoanode = +0.34 – –1.18 = +1.52 V
Thus +1.52 V is the correct answer.
Which combination would electroplate an object with nickel?
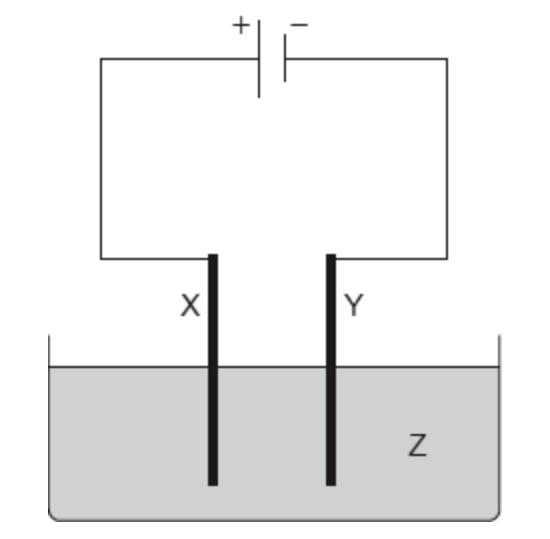
If electrodes are inert (e.g. platinum) then they do not get involved in the electrolysis process. Electrodes that are made of other metals may be preferentially involved in the electrolysis, depending on position in the electrochemical series.
If nickel electrodes (instead of inert electrodes) are used to electrolyse nickel sulfate (NiSO4(aq)) solution then nickel will be lost from the anode (positive electrode) and gained at the cathode (negative electrode).
Therefore the object needs to be placed as the cathode (Y) as nickel is deposited on the cathode, and the anode (X) needs to be nickel, as nickel is lost from the anode (instead of oxygen being produced). The solution needs to be a nickel salt solution, in this case NiSO4(aq).
Thus Electrode X: Nickel Electrode Y: Object Electrolyte Z: NiSO4(aq) is the correct answer.
Two molten electrolyte cells are electrolysed in series. What mass of magnesium (Ar = 24) is deposited at the cathode in ‘cell A’, if \(x\) g of gallium (Ar = 70) is deposited at the cathode in ‘cell B’?
Moles of gallium = mass/Ar = \(x \over 70\)
Half equations: Ga3+ (aq) + 3e– → Ga (s) and Mg2+ (aq) + 2e– → Mg (s) so ratio of moles of electrons for gallium to moles of electrons for magnesium is 3:2.
This means that the ratio of moles of gallium : magnesium will be 2:3 (since the same number of electrons will pass through the circuit, but that will give more magnesium atoms as only two electrons are needed for each atom, rather than 3 for gallium).
Moles of magnesium = \(x \over 70\) × \(3 \over 2\)
Mass of magnesium = moles × Ar = \(x \over 70\) × \(3 \over 2\) × 24
Which multiplied out gives \(72x \over 140\) which is therefore the correct answer.
Which statements about a voltaic cell is/are correct, given the overall cell reaction?
2Ag+ (aq) + Fe(s) → 2Ag (s) + Fe2+(aq) Eo = +1.25 V
1: Electrons flow from the iron half-cell to the silver half-cell.
2: The silver half-cell has a more positive standard electrode potential than iron.
3: Silver is reduced and iron is oxidised.
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) causing the electrode to be negative, and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction) causing the electrode to be positive.
Therefore the iron half-cell must be more negative (Eo) than the silver half-cell (since the iron loses electrons, which is oxidation) and the silver half-cell which gains electrons (reduction) is more positive (Eo). Electrons will flow from negative to positive; from iron to silver.
Thus 1, 2 and 3 is the correct answer.
A spontaneous redox reaction takes place when two half-cells are connected. What must be true about the redox reaction?
For any reaction to be spontaneous Gibbs Free Energy, ΔGo, must be negative.
And since ΔGo = −nFEocell (this should be remembered) the standard cell potential, Eocell , will always be positive for a spontaneous reaction.
Therefore, Eocell must be positive and ΔGo must be negative is the correct answer.
Which solution forms both hydrogen and oxygen at the electrodes when a concentrated solution is electrolysed using inert electrodes?
The species discharged at the cathode/negative electrode (gaining e–) will be lower (less –ve) in the electrochemical series. Without the data booklet we must remember that the least reactive metal/hydrogen is discharged.
Water is lower than potassium so water is discharged preferentially to potassium, forming hydrogen gas (hydrogen is lower in the activity series than potassium). Copper is lower than water so copper will be preferentially discharged over water.
The species discharged at the anode/positive electrode (losing e–) will be higher (less +ve) in the electrochemical series. Again, without the data booklet we must remember that the least reactive non-metal is discharged.
Note! Exception: Chloride ions (½Cl2(g) + e– ⇌ Cl–(aq) Eo = +1.36 V) are preferentially discharged over water (½O2(g) + 2H+(aq) + 2e– ⇌ H2O(l) Eo = +1.23 V) at the positive electrode despite their Eo values (this is a kinetic effect that needs remembering).
Water is preferentially discharged over compound ions (like SO4, NO3, etc.) at the anode/positive electrode, forming oxygen gas.
Thus K2SO4 (aq) is the correct answer, since water will be preferentially discharged at both cathode and anode, producing hydrogen and oxygen.
The overall equation for a voltaic cell is:
Sn (s) + Cu2+ (aq) → Sn2+ (aq) + Cu (s) Eocell = +0.48 V
Given the standard electrode potential for the copper half-cell, what is the standard electrode potential for the tin half-cell?
Cu2+(aq) + 2e– ⇌ Cu(s) Eo = +0.34 V
Sn2+(aq) + 2e– ⇌ Sn(s) Eo = ?
In a voltaic cell the half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction).
Red Cat: Reduction at cathode, An Ox: Oxidation at anode.

The cell potential is the difference (as on a number line) in the potential of the two half-cells.
Or mathematically Eocell = Eocathode – Eoanode
Therefore, Eoanode = Eocathode – Eocell = +0.34 – +048 = −0.14 V
Thus −0.14 V is the correct answer.
How much of Redox AHL (HL only) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn