Topics 4.1, 4.2, 4.3, 4.4 and 4.5
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which molecule is likely to be the most polar?
To be polar, a molecule must have polar bonds (differences in electronegativity) within it and must have asymmetric geometry (molecules will then have an overall dipole moment). If the dipoles of polar bonds cancel out because the molecule is symmetrical, then the molecule will have no overall dipole.
The Cδ+=Oδ– bonds in CO2 are polar, but the molecule is linear and this symmetry cancels out the dipoles, so CO2 is a non-polar molecule.
The Cδ+–Fδ– bonds in CF4 are polar, but the molecule is tetrahedral and this symmetry cancels out the dipoles, so CF4 is a non-polar molecule.
Both CH2F2 and CF2Cl2 will be polar as their geometry is asymmetrical and the carbon−halogen bonds are polar.
CH2F2 will be the most polar since the electronegativity difference between fluorine and hydrogen (the two 'sides' of the molecule) is greater than the electronegativity difference between fluorine and chlorine in CF2Cl2.
CH2F2 is therefore the correct answer.
What is the electron domain geometry and the molecular geometry of the carbonate ion, CO32−?
The Lewis structure of the carbonate ion, CO32− can be drawn with one double C=O bond and two single C−O bonds. It obeys the octet rule (and also exhibits resonance; not shown here):
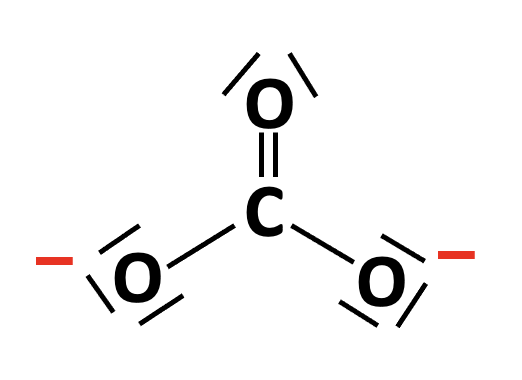
There are three electron domains (three bonds and no lone-pairs) around the central atom, so the electron domain shape is trigonal planar, and the molecular geometry (molecular shape) is also trigonal planar.
Electron domain: Trigonal planar Molecular: Trigonal planar is therefore the correct answer.
Which series shows the correct order of metallic bond strength from strongest to weakest?
The strength of a metallic bond relies largely on the nature of the metal ions; smaller and/or more highly charged ions will have stronger interactions with the delocalised sea of electrons (the electrons will be closer to the nuclear charge).
Aluminium (Al) forms 3+ ions and is small (period 3) so has the highest metallic bond strength.
Calcium (Ca) forms 2+ ions and is in period 4 (larger ions) so will have lower metallic bond strength than Al, but higher bond strength than potassium (K) , which is also in period 4 but forms only 1+ ions.
Potassium has a smaller atom (and forms a smaller ion) than Rubidium (Rb) in period 5, so Rb has the weakest metallic bond strength.
Al > Ca > K > Rb is therefore the correct answer.
What is the correct formula for magnesium phosphate?
The correct answer is Mg3(PO4)2. The other answers represent compounds that don't exist in a stable form.
Working out formulae with polyatomic ions is the same as with other ionic compounds (balancing electrons gained and lost) but remember not to confuse the number of atoms with the charge - e.g. the phosphate ion consists of 1 phosphorus atom, 4 oxygen atoms, and has an overall charge of 3–. Magnesium in group 2 (II) has a charge of 2+ on its ion.
Remember that brackets are essential with polyatomic ions.
You do need to learn these polyatomic ions:
| ammonium | NH4+ |
| hydroxide | OH– |
| nitrate | NO3– |
| hydrogen carbonate | HCO3– |
| carbonate | CO32– |
| sulfate | SO42– |
| phosphate | PO43– |
Which compound has the shortest C to O bond?
Triple bonds are stronger and shorter than double bonds which are stronger and shorter than single bonds due to the greater number of electrons in the bond.
C≡O in carbon monoxide is the shortest and the strongest, since the other C to O bonds are double (CO2 and CH3CHO) or single bonds (CH3CH2OH)
CO is therefore the correct answer.
Which describes a defining feature of a resonance structure?
Resonance diagrams can be drawn when a molecule cannot be accurately represented by a single Lewis diagram.
Resonance structures involve shifting around double bonds to different positions in a molecule. For example, the carbonate ion CO32– is said to exhibit resonance, because the shape of the compound ion with three C–O bonds of equal length cannot be represented by a conventional Lewis diagram. The resonance structures are shown below (showing bonds only for clarity; lone pairs are not shown):
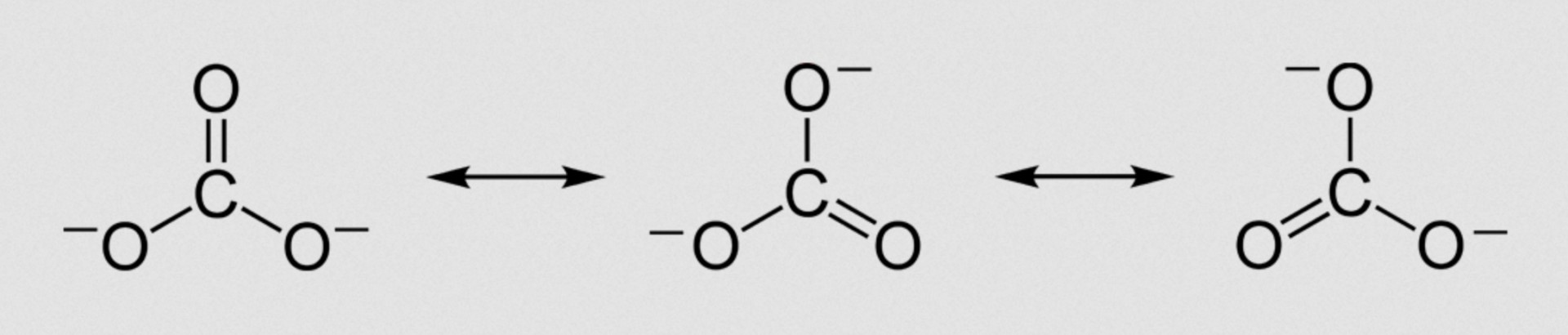
Resonance structures are not structural isomers (as the forms do not have different structural formulae), single bonds are never broken - only the positions of double bonds shift, and resonance structures may or may not include ionic as well as covalent bonding.
The correct answer is therefore Double bonds can be drawn in different positions.
Which statements are correct for all ionic compounds?
1: They conduct electricity when solid
2: They have high melting points
3: They form giant (lattice) structures
Ionic compounds have high melting and boiling points; they have giant (lattice) structures and it takes a lot of energy to break the network of strong ionic bonds. Ionic compounds do not conduct electricity in the solid state (ions cannot move) but do conduct when molten or in aqueous solution (ions can move and carry charge).
Therefore, 2 and 3 only is the correct answer.
Which species does not have resonance structures?
Species exhibit resonance when they cannot be represented by a single Lewis diagram.
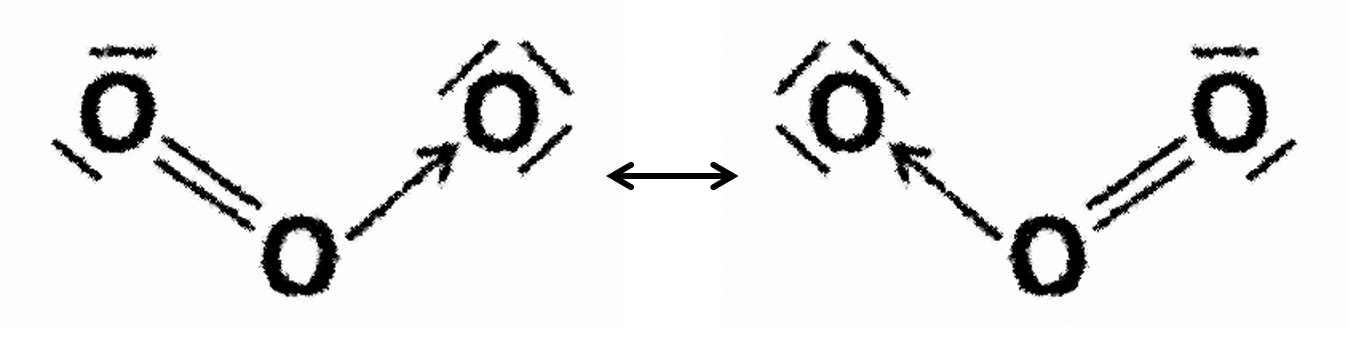
Ozone, O3, the carbonate ion, CO32–, and benzene, C6H6 all exhibit resonance; double bonds in these species can be pushed around the structures (see example below).
Ethene, C2H4, does not exhibit resonance, as it can be accurately represented by a single Lewis structure (and the double bond in ethene cannot be pushed anywhere else in the structure).
C2H4 is therefore the correct answer.
E.g. resonance in ozone:
What is the order of increasing boiling point?
All the molecules have similar masses (72 to 76) so the strength of the London dispersion forces that they all possess are likely to be very similar.
The accepted relative strengths of intermolecular forces are that hydrogen bonding is greater in strength than dipole-dipole forces that are in turn greater in strength than London dispersion forces (Hydrogen bonding > dipole-dipole forces > London dispersion forces).
Pentane (CH3(CH2)3CH3) is non-polar and has only London dispersion forces.
Butanone (CH3COCH2CH3) has polar bonds and is asymmetric so it has dipole-dipole forces between molecules as well as London dispersion forces.
Butan-1-ol (CH3CH2CH2CH2OH) has an O–H bond so it has hydrogen bonds between molecules as well as dipole-dipole forces and London dispersion forces.
Propan-1,3-diol (CH2OHCH2CH2OH) has two O–H bonds, so will have an even greater number of hydrogen bonds between molecules as well as dipole-dipole forces and London dispersion forces.
Thus CH3(CH2)3CH3 < CH3COCH2CH3 < CH3CH2CH2CH2OH < CH2OHCH2CH2OH is the correct answer.
Which molecule contains an incomplete octet of electrons?
The octet rule is based on the Bohr model of the atom, with a full outer shell containing 8 electrons (2 for the first shell).
Boron is in group III (13) so will have 3 electrons in the outer shell, combined with the 3 electrons from the three hydrogen atoms results in BH3 having only 6 electrons in its outer shell.
NH3, H2S and PH3, following the same logic, all have complete outer shells of 8 electrons.
BH3 is therefore the correct answer.
Which of the following substances when pure will form hydrogen bonds between its molecules?
1: C2H5OH
2: C2H6
3: CH3CHO
Molecules with a hydrogen atom attached to F, O or N possess hydrogen bonding between molecules.
Ethanol (C2H5OH) molecules will form hydrogen bonds with each other as they have O–H bonds.
Ethane (C2H6) and ethanal (CH3CHO) cannot form hydrogen bonds between molecules as they do not have an H atom attached directly to an oxygen atom (the functional group in an aldehyde is a carbonyl group, C=O with no H attached to the oxygen).
Hence the correct answer is 1 only.
What is the correct formula for chromium(III) hydroxide?
The correct answer is Cr(OH)3. The other answers represent compounds that don't exist in a stable form.
For d-block elements the oxidation state or charge on the ion is represented by the Roman numeral in the name; chromium(III) here indicates a Cr3+ ion.
Working out formulae with polyatomic ions is the same as with other ionic compounds (balancing electrons gained and lost).
Remember that brackets are essential with polyatomic ions, otherwise the number of atoms is misrepresented.
You do need to learn these polyatomic ions:
| ammonium | NH4+ |
| hydroxide | OH– |
| nitrate | NO3– |
| hydrogen carbonate | HCO3– |
| carbonate | CO32– |
| sulfate | SO42– |
| phosphate | PO43– |
How many lone pairs and bonding pairs of electrons surround the central sulfur atom in H2S?
The Lewis structure of hydrogen sulfide, H2S obeys the octet rule:
.png)
Sulfur, in group VI (16) has six electrons in its outer shell and each hydrogen contributes 1 electron, totalling 8 electrons.
There are four electron domains (two bonds and two lone-pairs) around the central atom (so the electron domain shape is tetrahedral, and the molecular geometry (molecular shape) is bent or non-linear).
2 lone pairs; 2 bonding pairs is therefore the correct answer.
Look at the table below:
Chemical | Melting/boiling point | Solubility in water | Electrical conductivity when molten |
| Z | low | high | high |
| Y | high | high | high |
| X | high | low | nil |
| W | low | low | nil |
Which of the four chemicals is most likely to have a giant covalent structure?
Giant covalent substances are likely to have high melting and boiling points; they have giant (lattice) structures and it takes a lot of energy to break the network of strong covalent bonds.
Giant covalent substances tend to be insoluble in water and do not tend to conduct electricity in the solid or molten state (graphite is an exception) as they do not possess ions or delocalised electrons that can carry charge.
X is therefore correct answer.
How much of Bonding and Structure core (SL and HL) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn