Topics 14.1 and 14.2
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which contain delocalised electrons?
1: C6H5CH3
2: CO32−
3: HCOO−
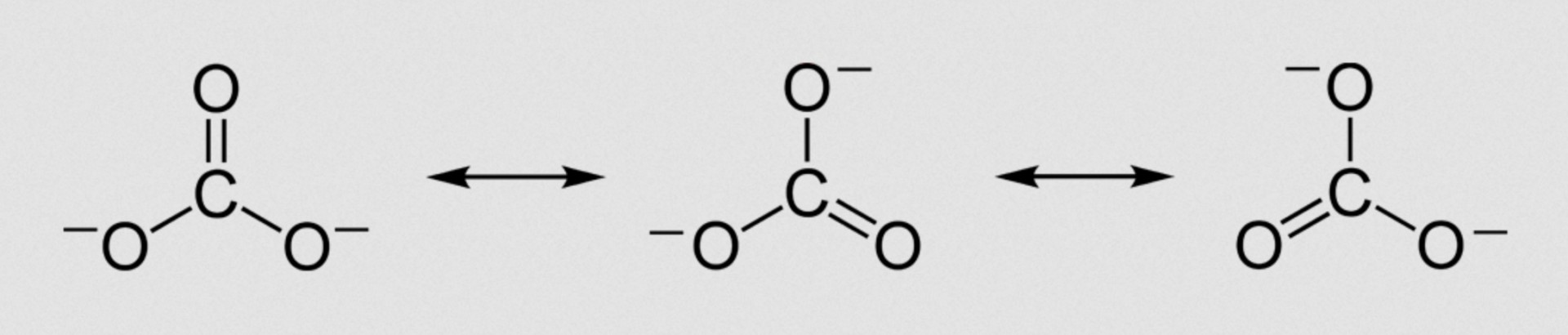
Species exhibit delocalised electrons (or resonance) when they cannot be represented by a single Lewis diagram and double bonds can be pushed around the structures (see example below).
The benzene ring (phenyl group C6H5) in methyl benzene, C6H5CH3, does have delocalised electrons (and exhibits resonance). The carbonate ion and the methanoate ion also both have delocalised electrons (and exhibit resonance).
1, 2 and 3 is therefore the correct answer.
E.g. delocalisation/resonance in carbonate ion:
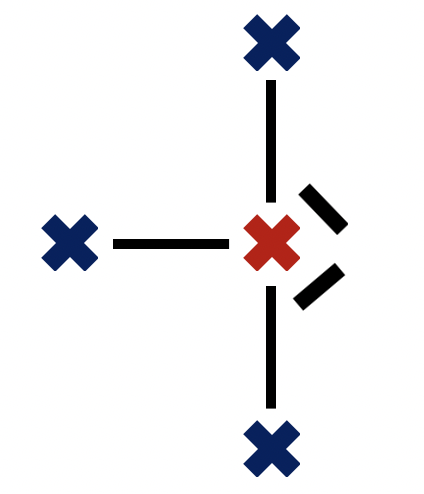
Which correctly describes the molecular geometry in BrF3?
Bromine trifluoride, with three bonding pairs and two lone-pairs around the central bromine atom is T–shaped, which is therefore the correct answer.
The shape of the electron domains is trigonal bi-pyramidal, but with two equatorial (rather than axial) vertices missing (the lone pairs) the bonds form the shape of a 'T'.
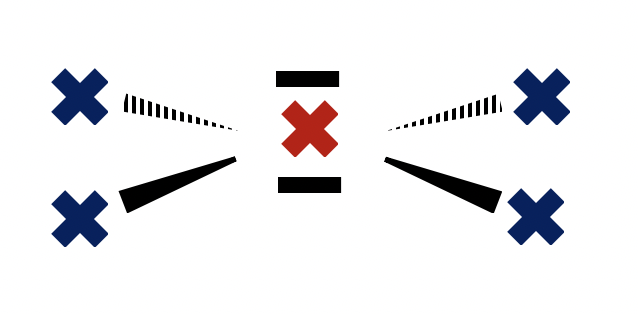
Which correctly describes the electron domain geometry and the molecular geometry, respectively, in BrCl4− ?
BrCl4− with four bonding pairs and two lone-pairs around the central bromine atom is square planar in molecular shape.
The shape of the electron domains is octahedral, but with two vertices missing (the lone pairs) the bonds form the shape of a square.
Thus, Octahedral and Square planar is the correct answer.
Which atom is sp2 hybridized?
When identifying the hybridization of a given atom, it is best to use our 'rule-of-thumb'; that the geometry of the electron domains (see molecular shapes in the covalent structure section 4.3) tells us the hybridization:
Tetrahedral electron domains means sp3 hybridization.
Trigonal planer electron domains means sp2 hybridization.
Linear electron domains means sp hybridization.
The carbon atom in H2CO has electron domains that are arranged in a trigonal planar manner (two single bonds and a double bond) so it must be sp2 hybridized.
Carbon in H2CO is therefore the correct answer.
(In CO2 the electron domain shape around carbon is linear, so sp hybridized; In CO the electron domain shape around oxygen is linear - a triple bond - so sp hybridized; In NH3 the electron domain shape around nitrogen is tetrahedral, so sp3 hybridized.)
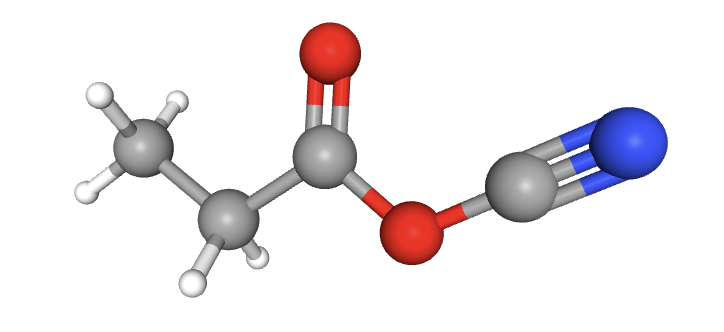
How many carbon atoms (grey) are sp3, sp2 and sp hybridized in the molecule shown?
When identifying the hybridization of a given atom, it is best to use our 'rule-of-thumb'; that the geometry of the electron domains (see molecular shapes in the covalent structure section 4.3) tells us the hybridization:
Tetrahedral electron domains means sp3 hybridization.
Trigonal planar electron domains means sp2 hybridization.
Linear electron domains means sp hybridization.
In order as shown, the electron domain shapes around the carbon atoms are: tetrahedral; tetrahedral; trigonal planar; linear. Thus, the hybridization is sp3; sp3; sp2; sp.
2 sp3 carbons; 1 sp2 carbon; 1 sp carbon is therefore the correct answer.
How many sigma (σ) and pi (π) bonds are there in ethanitrile (CH3CN)?
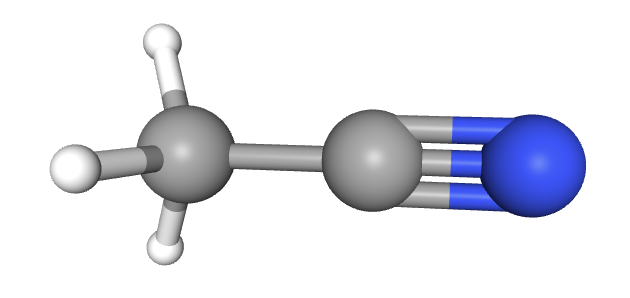
There are 7 bonds in the molecule. Every bond is one pair of electrons. Every sigma bond and every pi bond is one pair of electrons.
A single bond is just a sigma bond.
A double bond is one sigma bond and one pi bond.
A triple bond is one sigma and two pi bonds.
The correct answer is therefore 5 sigma bonds and 2 pi bonds present in ethanitrile.
What is the hybridization of the oxygen and two nitrogen atoms circled?
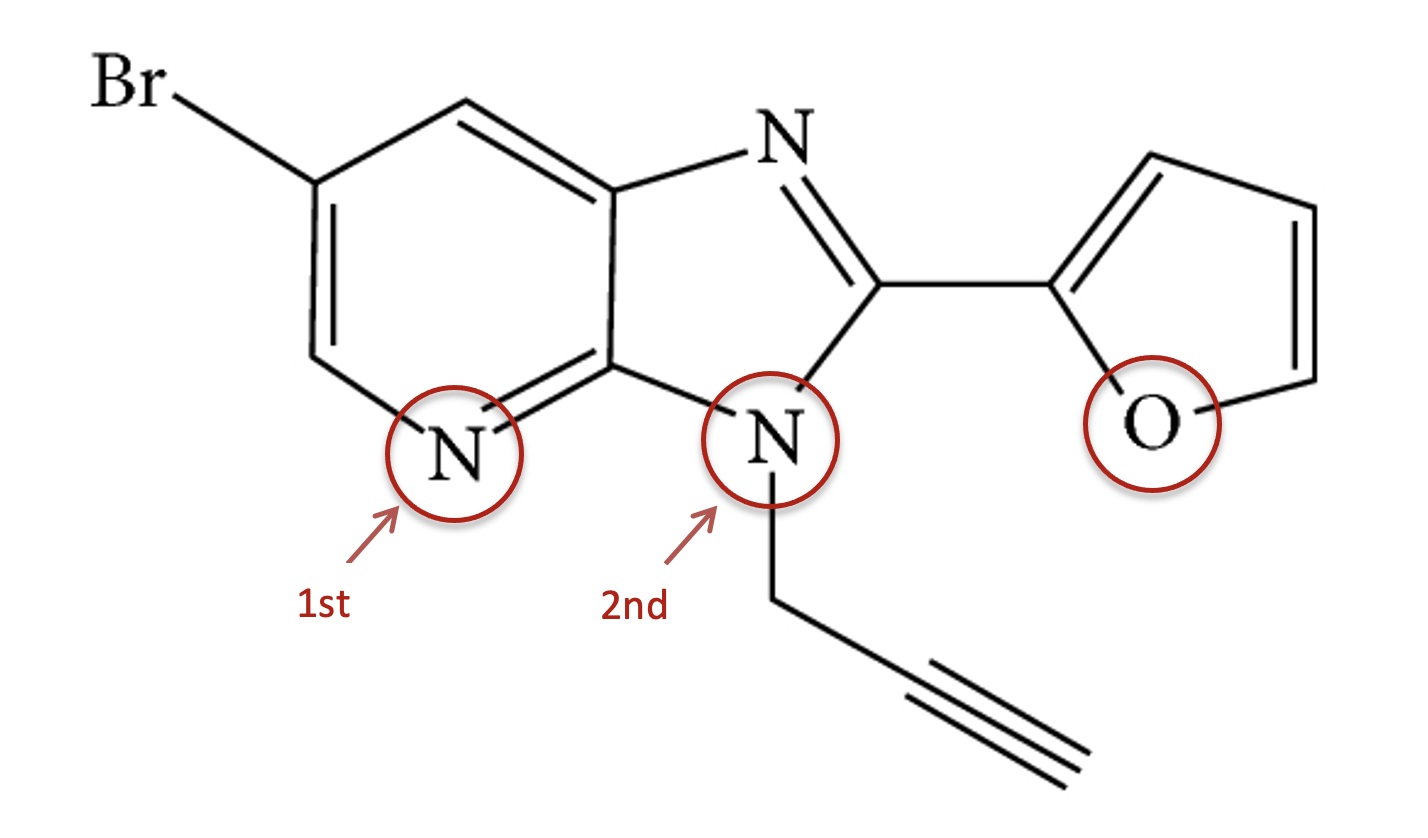
When identifying the hybridization of a given atom, it is best to use our 'rule-of-thumb'; that the geometry of the electron domains (see molecular shapes in the covalent structure section 4.3) tells us the hybridization:
Tetrahedral electron domains means sp3 hybridization.
Trigonal planar electron domains means sp2 hybridization.
Linear electron domains means sp hybridization.
In order as shown:
1st N electron domain shape is trigonal planar (don't forget the N atom has a lone pair). Thus, the hybridization is sp2.
2nd N electron domain shape is tetrahedral (don't forget the N atom has a lone pair). Thus, the hybridization is sp3.
O electron domain shape is tetrahedral (don't forget the O atom has two lone pairs). Thus, the hybridization is sp3.
1st N is sp2 ; 2nd N is sp3 ; O is sp3 is therefore the correct answer.
Which species has a square pyramidal molecular geometry?
Sketching the Lewis structures will allow us to determine that:
Bromine pentafluoride has a square pyramidal molecular geometry and BrF5 is therefore the correct answer.
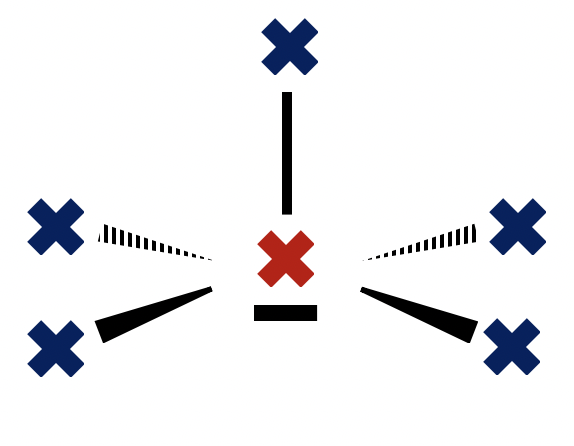
BrF4− is square planar.
PF5 is trigonal bipyramidal.
SiH4 is tetrahedral.
How many sigma (σ) and pi (π) bonds are present in this molecule?
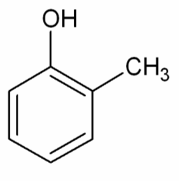
Don't forget that there are single bonds between the OH and the CH3 atoms.
There are 9 single bonds and 3 double bonds.
So, there are 15 bonds in the molecule; every bond is one pair of electrons. Every sigma bond and every pi bond is one pair of electrons.
A single bond is just a sigma bond.
A double bond is one sigma bond and one pi bond.
A triple bond is one sigma and two pi bonds.
The correct answer is therefore 12 sigma bonds and 3 pi bonds present in the molecule.
Which species does not have a bond angle of 90°?
Sketching the Lewis structures will allow us to determine that:
SF6 is octahedral; SF4 has a seesaw shape; XeF4 is square planar. These all have (approx.) 90° bond angles.
GeF4 is tetrahedral (since Ge is in group 14) and so will have 109.5° bond angles, and not have a bond angle of 90°.
GeF4 is therefore the correct answer.
How much of Bonding and Structure AHL (HL only) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn