Topics 5.1, 5.2 and 5.3
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Using the standard enthalpies of combustion given below, what is enthalpy change for the following reaction?
C7H16 (l) → C2H4 (g) + C5H12 (l)
ΔHc°(C7H16 (l)) = −4817 kJ mol−1
ΔHc°(C2H4 (g)) = −1411 kJ mol−1
ΔHc°(C5H12 (l)) = −3509 kJ mol−1
We are given ΔH combustion data so we construct the energy cycle by linking the reactants and products through the combustion products (arrows point down to combustion products):
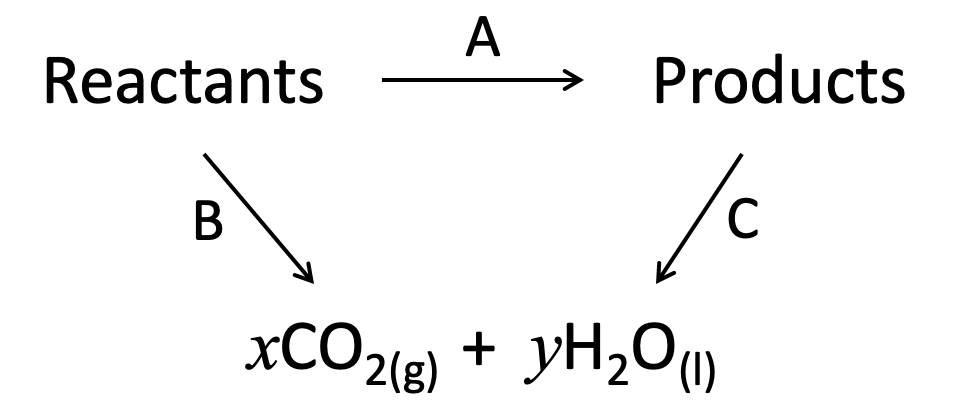
The enthalpy change for the reaction given (A) can then be found: The alternative route going via the combustion products is therefore A = B−C (−C because we are going against the arrow so the sign must be inverted).
All changes are for one mole of combustion, so the calculation is as follows:
B = −4817
C = (−1411)+(−3509) = −1411−3509
A = B−C = −4817−(−1411−3509) = −4817+1411+3509 which rearranged in the order given in the answer gives + 1411 + 3509 − 4817
The correct answer is therefore + 1411 + 3509 − 4817
Which equation represents the P−H bond enthalpy in PH3?
Bond enthalpy is defined as the average enthalpy required to break one mole of covalent bonds in the gaseous state.
So the best representation is going to be a chemical change showing the average breaking of one mole of P−H bonds in the gaseous state.
PH3(g) → PH2(g) + H(g) shows a single P−H bond breaking and by definition (stoichiometric coefficients) we have one mole of bonds breaking. But, when we break the bonds in any molecule one-by-one the bonds will all have different enthalpies as the environment of the bonds is changing.
Therefore we need to break all three bonds in PH3 and find the average enthalpy:
⅓PH3(g) → ⅓P(g) + H(g) is thus the correct answer.
Incorrect answers
PH3(g) → P(g) + 3H(g) and PH3(g) → P(g) + 1½H2(g) both show three moles of P−H bonds breaking, with the hydrogen products shown as atoms or molecules.
When equal masses of X and Z absorb the same amount of energy their temperatures rise by 10°C and 20°C respectively.
Which is correct?
Specific heat capacity is the amount of energy needed (J) to raise one unit of mass (g) by one unit of temperature (K or °C - the interval/unit size is the same so either if fine). E.g. the specific heat capacity of water is 4.18 JK−1g−1
In this question the same amount of energy raises X by 10°C and Z by 20°C. So it takes more energy to raise the temperature of X than Z. In fact, as the relationship between mass and energy needed to raise the temperature is linear, it must take twice as much energy to raise X by 20°C. So X must have twice the specific heat capacity of Z.
The specific heat capacity of X is twice that of Y is therefore the correct answer.
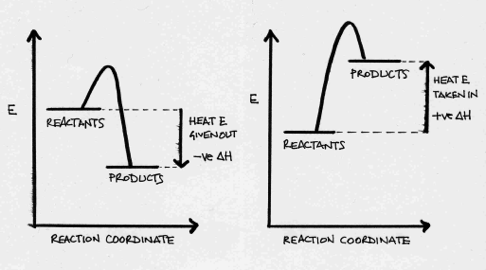
Which is correct for the reaction?
2NO2 → N2O4 ΔH = −57.6 kJ mol−1
A negative ΔH indicates that the reaction is exothermic.
If a reaction is exothermic, then heat energy (enthaply) is transferred from the system to the surroundings. Thus the system loses energy; ΔH is negative, and the energy of the products is lower than the energy of the reactants; so the products are more stable than the reactants.
Thus, the products are more stable than the reactants and the reaction is exothermic is the correct answer.
On the left; exothermic - on the right; endothermic.
What is the enthalpy change for the incomplete combustion of ethene using average bond enthalpies given below.
C2H4 (g) + 2O2 (g) → 2CO (g) + 2H2O (g)
Bond enthalpies (in kJ mol−1) that you may require: C−H 414 / C=C 614 / O=O 498 / C≡O 1077 / O−H 463
Total energy change = bonds broken − bonds made
Bonds present in reactants: 1×C=C, 4×C−H, 2×O=O
Bonds present in products: 2×C≡O, 4×O−H
Assuming all the bonds in reactants are broken and all bonds in products are made:
Enthalpy for bonds broken: 614 + 4(414) + 2(498)
Enthalpy for bonds made: 2(1077) + 4(463)
Thus the correct answer is [614 + 4(414) + 2(498)] − [2(1077) + 4(463)]
Using the standard enthalpies given below, what is the standard enthalpy of combustion of ethane in kJ mol−1?
2C2H6 (g) + 7O2 (g) → 4CO2 (g) + 6H2O (l)
C (s) + O2 (g) → CO2 (g) = X kJ mol−1
H2 (g) + ½O2 (g) → H2O (l) = Y kJ mol−1
2C (s) + 3H2 (g) → C2H6 (g) = Z kJ mol−1
We are given ΔH formation data so we construct the energy cycle by linking the reactants and products through the elements (arrows point up from elements):
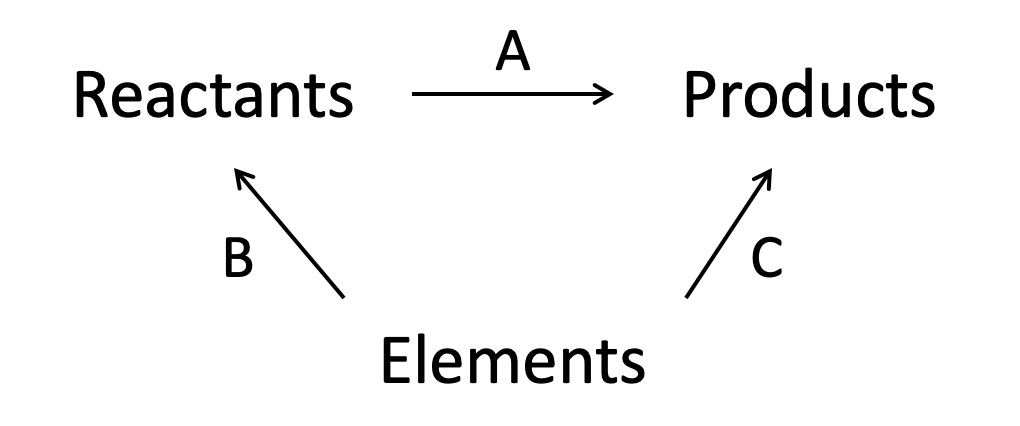
The enthalpy change for the reaction given (A) can then be found: The alternative route going via the elements is therefore A = −B+C (−B because we are going against the arrow so the sign must be inverted).
As written in the question, the changes are:
B = 2Z (O2 is already an element so there is no change)
C = 4X + 6Y
A = −B+C = −2Z + 4X + 6Y
or rearranged: 4X + 6Y − 2Z
But this is for two moles of ethane! The standard enthalpy of combustion is always per mole (hence units kJ mol−1).
The correct answer is therefore 2X + 3Y − Z
Two 50.0 cm3 aqueous solutions containing 0.020 mol of hydrochloric acid and 0.020 mol of sodium hydroxide respectively are both at 298K.
When the two solutions are mixed the temperature rises by y °C.
Assume that the density of the final solution is 1.00 g cm−3 and the specific heat capacity is 4.18 J K−1 g−1.
What is the enthalpy change of neutralisation in kJ mol−1?
Using Q=mcΔT.
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, the final solution). The final solution is assumed to have a density of 1.00 g cm−3 (so 100cm3 is 100g; that is the two solutions poured together).
Q = 100 × 4.18 × y Joules
The enthalpy change of neutralisation must be given per mole (of HCl or NaOH) and in kJ (not in Joules).
Dividing by 0.020 will give the value per mole of HCl (or NaOH), and dividing by 1000 will give the value in kJ mol−1.
Thus the answer is \({100 \times 4.18 \times y \over 1000 \times 0.020}\)
Which expression gives the mass, in g, of propan-1-ol required to produce 790 kJ of heat energy upon complete combustion?
(Mr for propan-1-ol = 60.1, ΔHoc = −2021 kJ mol−1)
One mole of propan-1-ol produces 2021 kJ of heat energy and has a molar mass of 60.1g.
The fraction of one mole needed to produce 790 kJ of energy will therefore be \({790 \over 2021}\)
Therefore the mass of propan-1-ol needed will be the mole fraction × molar mass, which is \({790 \over 2021}\) × 60.1
Thus the answer is \({790 \times 60.1 \over 2021}\)
Why is the enthalpy change for this reaction calculated from bond enthalpy data less accurate than that calculated using standard enthalpies of formation?
C3H6 (g) + 4½O2 (g) → 3CO2 (g) + 3H2O (g)
Bond enthalpy is defined as the average enthalpy required to break one mole of covalent bonds in the gaseous state.
All of the species in this equation are gaseous, so that is not a problem for a bond enthalpy calculation. However, since bond enthalpies are average values, the enthalpy change calculated using bond enthalpies will not be accurate to this reaction.
Therefore the correct answer is Bond enthalpies are average values of many compounds.
What can be deduced from the knowledge that ozone absorbs ultraviolet radiation of a greater wavelength than oxygen?
Both oxygen and ozone will absorb ultraviolet radiation in the atmosphere and the bonds of both molecules may break to form oxygen free radicals. Ozone absorbs energy of a greater wavelength. Wavelength is inversely proportional to frequency and hence energy, so ozone absorbs radiation of a lower energy.
The double bond in oxygen (O2) is therefore stronger and requires more energy to break than the '1½' bonds in ozone (O3).
The correct answer is therefore The bonds between atoms in oxygen require more energy to break than those in ozone
What is the enthalpy of formation of carbon monoxide, in kJ mol−1, as represented by ΔH3?
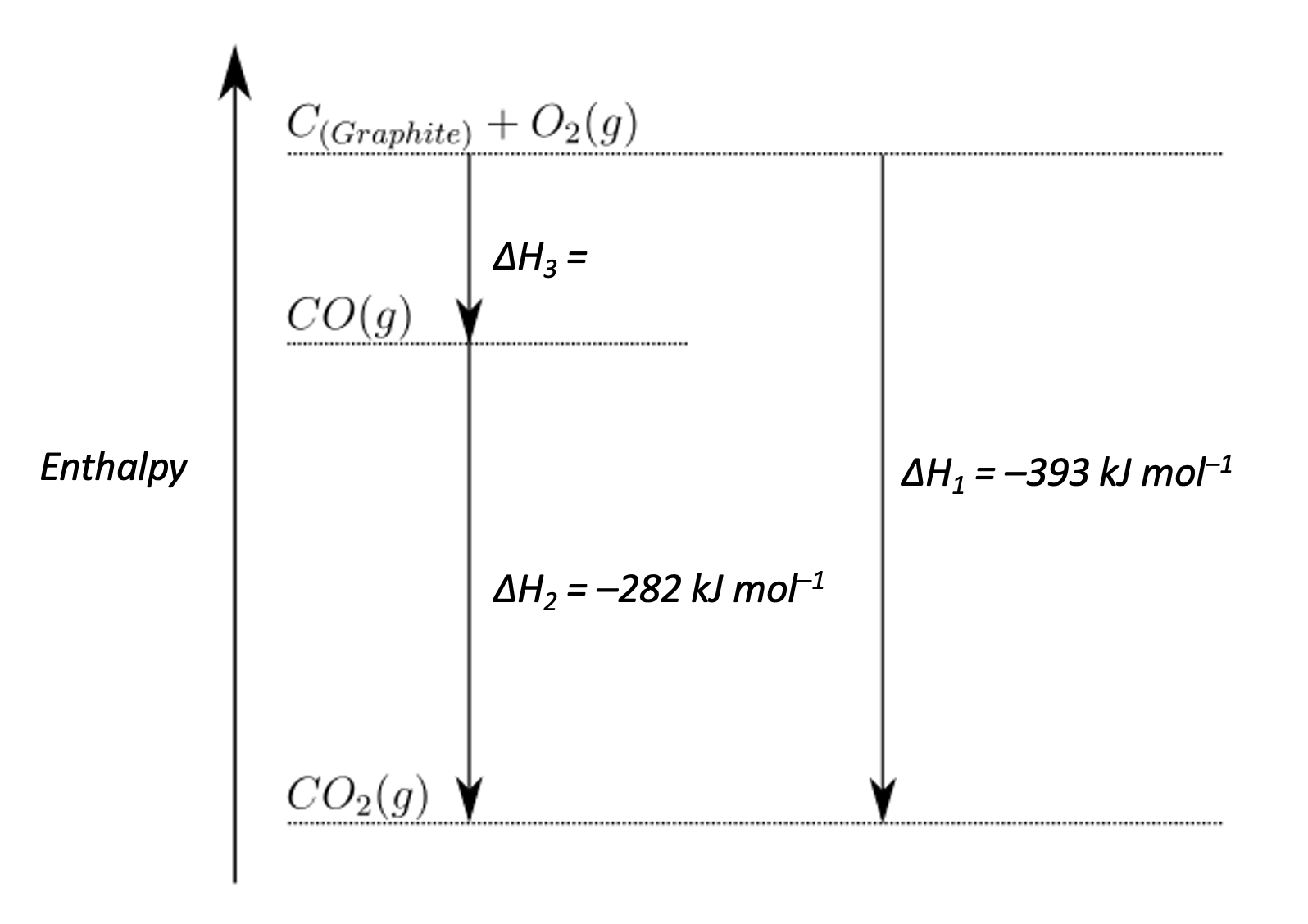
ΔH3 is the enthalpy change from elements to carbon monoxide. An alternative route is to go from elements to carbon dioxide and then back up to carbon dioxide. This alternative route 'goes with' the first arrow of the enthalpy change ΔH1 so the sign (indicating the exothermic or endothermic nature) of the enthalpy change is unchanged. And this alternative route then 'goes against' the second arrow of the enthalpy change ΔH2 so the sign (indicating the exothermic or endothermic nature) of the enthalpy change is inverted (minus-minus is a plus).
Therefore the enthalpy change is −393−(−282) = 282−393
Thus, the correct answer is 282−393
5.85g of solid sodium chloride, NaCl(s), was added to water to form 25.0g of solution. The maximum decrease in temperature was 3.7°C.
What is the enthalpy change, in kJ mol−1 for this process?
(Assume that the specific heat capacity of the solution is 4.18 J K−1 g−1 and the molar mass of NaCl is 58.5 g mol−1.)
Using Q=mcΔT.
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, the resulting solution).
Q = 25.0 × 4.18 × 3.7 Joules
The enthalpy change must be given per mole and in kJ (not in Joules).
Moles of sodium chloride = \({5.85 \over 58.5}\)= 0.100 (or 0.1 for simplicity)
Dividing by 0.1 will give the value per mole of NaCl, and dividing by 1000 will give the value in kJ mol−1.
The temperature decreases so the reaction is endothermic, and therefore ΔH is positive.
Thus the answer is \(\Delta H=+ {25\times 4.18 \times 3.7 \over 1000 \times 0.1}\)
How much of Energetics core (SL and HL) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn