Topic 21.1
Paper 1 style questions are multiple choice. You are not permitted to use a calculator or the data book for these questions, but you should use a periodic table.
A periodic table pop-up is available on the left hand menu.
Which technique might be used to determine the bond lengths and bond angles in solid benzene?
X-ray crystallography is a method of determining crystal structures. X-rays are fired at a crystal sample and diffract to create three dimensional patterns and pictures that crystallographers can read and decipher to give information about the arrangements of particles and electron density in substances:
Bond lengths and bond angles are determined with x-ray crystallography.
The other techniques give a lot of information about the structure of organic compounds, but do not tend to give direct information about bond lengths and angles.
X-ray crystallography is the correct answer.
Which can be identified in a molecule using infrared (IR) spectroscopy?
Infrared (IR) spectroscopy is used to identify the bonds that are present in a molecule. This allows the identification of potential functional groups.
The presence of a carbonyl group is therefore the correct answer.
The number of hydrogen environments could be determined by 1H-NMR; The molecular mass by Mass Spectrometry; Bond lengths and angles by X-ray Crystallography.
Which technique would be the most effective method to distinguish between 2-chloropropane and 1-chloropropane?
1H-NMR spectroscopy is the correct answer, as this would be the most effective technique: 1-chloropropane would exhibit three hydrogen environments (3:2:2) and 2-chloropropane would exhibit two hydrogen environments (6:1) as it is symmetrical (also the splitting patterns would be very different).
Infrared (IR) spectroscopy is used to identify the bonds that are present in a molecule. This allows the identification of potential functional groups, but would not easily distinguish between 2-chloropropane and 1-chloropropane.
Mass spectrometry, in the hands of skilled practitioner could be used, as the fragmentation patterns of the molecules would be different, but it would not be the most effective technique.
Reaction with silver nitrate solution, AgNO3(aq) and ethanol, would generate a white precipitate (AgCl(s)) but would not distinguish between 2-chloropropane and 1-chloropropane.
Which compound gives this 1H-NMR spectrum?
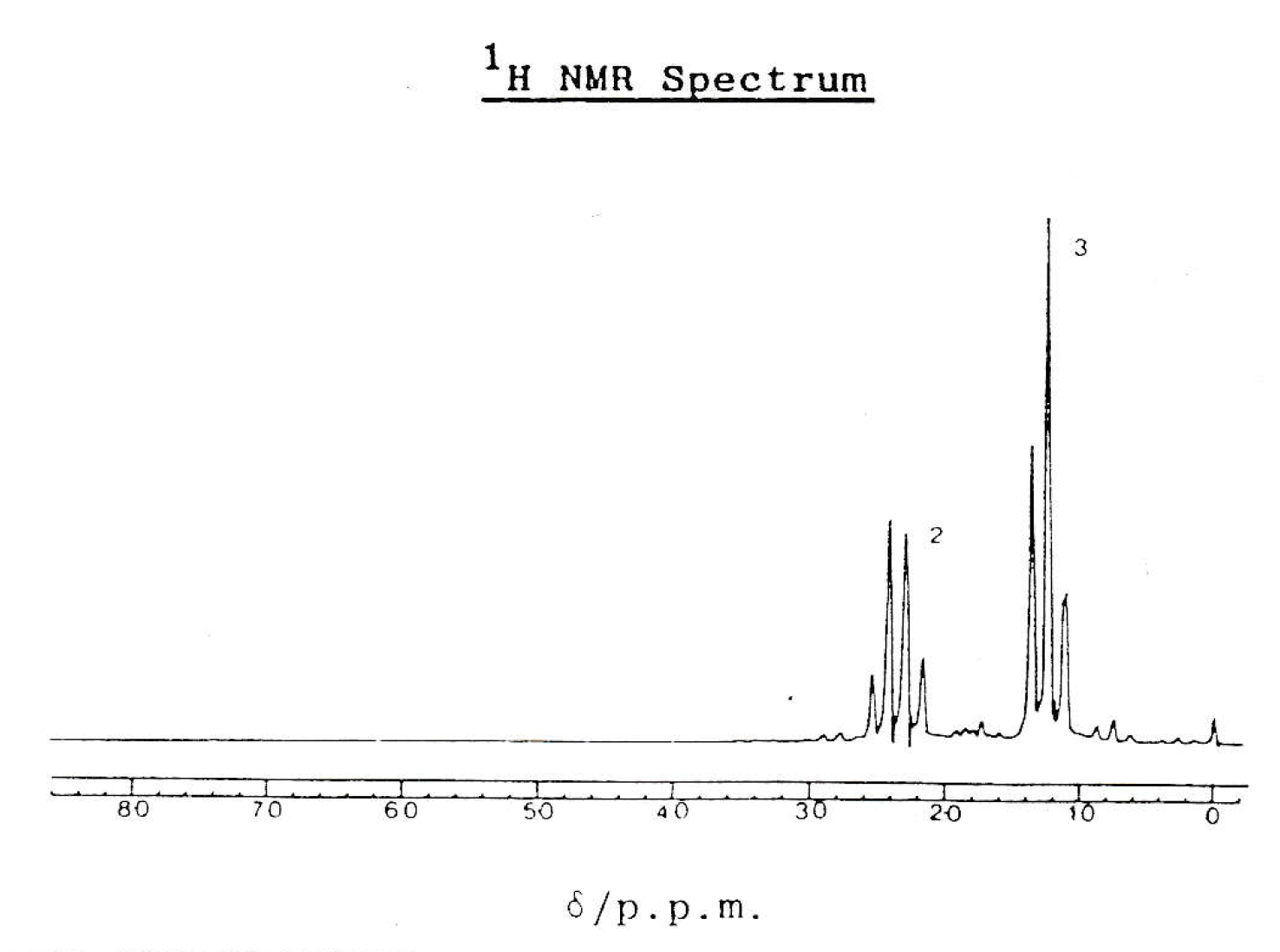
There are two peaks in the spectrum, so the compound has two hydrogen environments.
The peak at approximately 1.2ppm is a triplet because it is split by two nearest neighbours (according to the n+1 rule); presumably a CH2 group on the next carbon.
The peak at approximately 2.4ppm is a quartet because it is split by three nearest neighbours (according to the n+1 rule); presumably a CH3 group on the next carbon.
Propanenitrile (CH3CH2CN) has three hydrogens in the CH3 group that will split the neighbouring hydrogen environment (CH2 group) at 2.4ppm into a quartet, and vice versa to give the triplet at 1.2ppm.
Thus CH3CH2CN is the correct answer.
CH3CH2OH and CH3CHCH2 both have three hydrogen environments. CH3COCH2Cl has two hydrogen environments, but they are separated by an C=O group, are therefore not neighbouring, so would not split one-another.
Which compound produced this 1H-NMR spectrum?
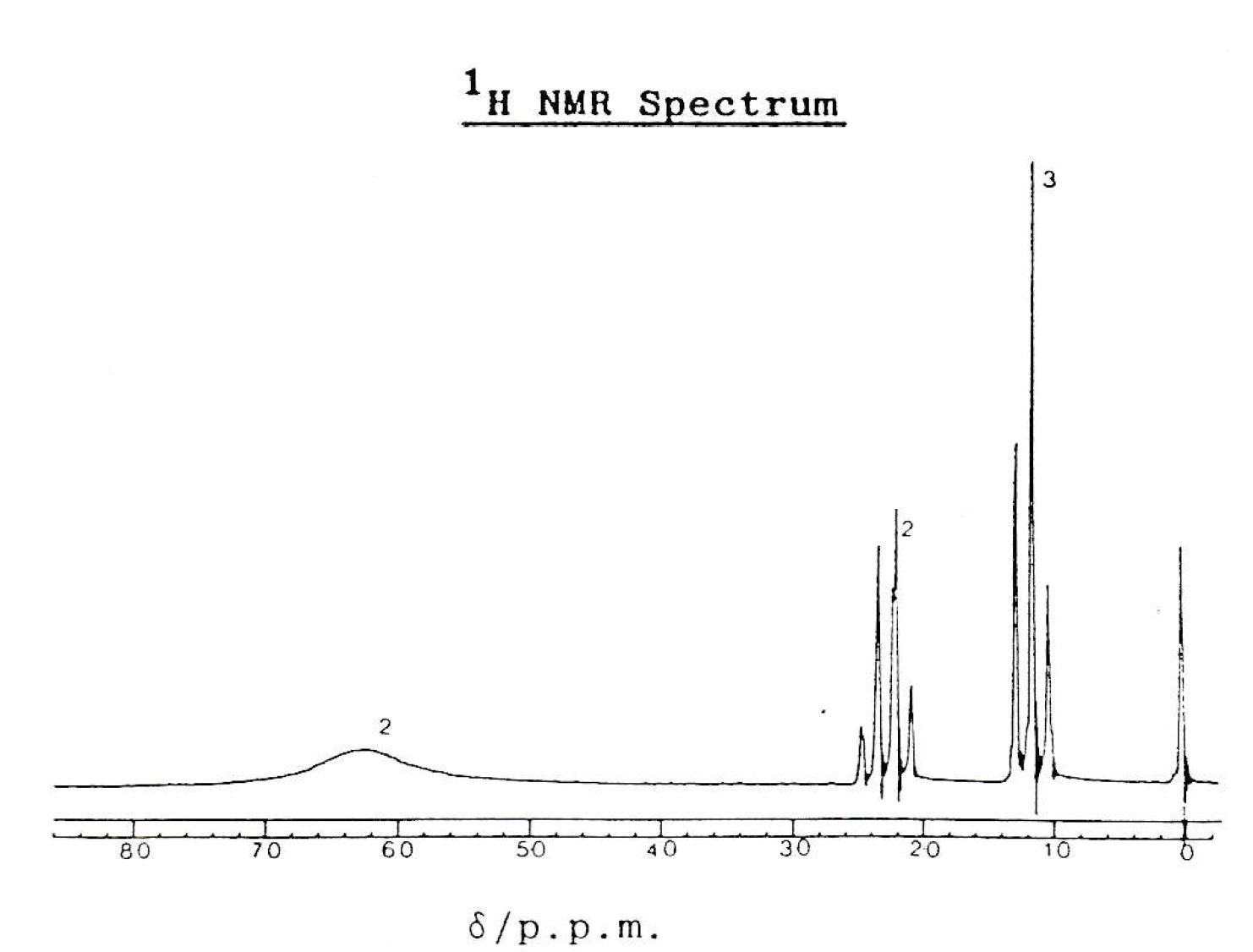
The NMR spectrum shows three hydrogen environments, with hydrogen ratio of 2:2:3. The splitting pattern suggests that the CH2 and CH3 are neighbouring (split into a quartet and triplet respectively), but the two protons at ppm 6.2 are not split by neighbouring protons.
Therefore CH3CH2CONH2 is the correct answer.
CH3CH2CH2OH has four hydrogen environments; CH3CH2CH2Cl would have a different splitting pattern (all environments would be split as they are all neighbouring); CH3CO2CH2CH3 would show peak integrals of 3:2:3 (rather than 2:2:3).
Which are true statements about what 1H-NMR spectra can always tell us about a molecule?
1: The total number of hydrogen atoms
2: The number of hydrogen atoms attached to adjacent atoms
3: The total number of hydrogen environments
1H-NMR spectroscopy summary:
Peaks: The number of peaks tells us the number of different hydrogen environments.
Ratio: The integration trace or whole number written adjacent to each peak tells us the ratio of the number of hydrogens in each environment.
Splitting: The spin-spin splitting pattern of each peak tells us the number of hydrogens in neighbouring environments (on adjacent atoms).
Shift: The chemical shift table (data book) can help us with the types of hydrogens (functional groups etc.) in the molecule.
Therefore 2 and 3 only is the correct answer. Since 1H-NMR will not always tell us the total number of hydrogen atoms in a molecule (as sometimes we aere just given the ratio).
Which of these properties contribute to why tetramethylsilane (TMS) is used as a reference standard for 1H-NMR?
1: The protons are all in the same environment
2: The chemical shift is low relative to other proton environments
3: It has high chemical reactivity
Tetramethylsilane, (CH3)4Si, is used as a reference for 1H-NMR spectroscopy because:
- All 12 hydrogen atoms are in the same environment so it gives one large sharp peak.
- The chemical shift is low (set as zero) relative other proton environments.
- It is unreactive, so does not intefere with the sample being tested.
- It is volatile (evaporates easily) so can easily be removed from a sample.
Therefore 1 and 2 only is the correct answer.
Which are correct statements about the 1H-NMR spectrum of propan-2-ol?
1: There will be four peaks in the spectrum (four hydrogen environments)
2: The integration trace will show a ratio of 1:1:6
3: There will be a quartet peak in the spectrum
The 3D image of propan-2-ol in shown below.
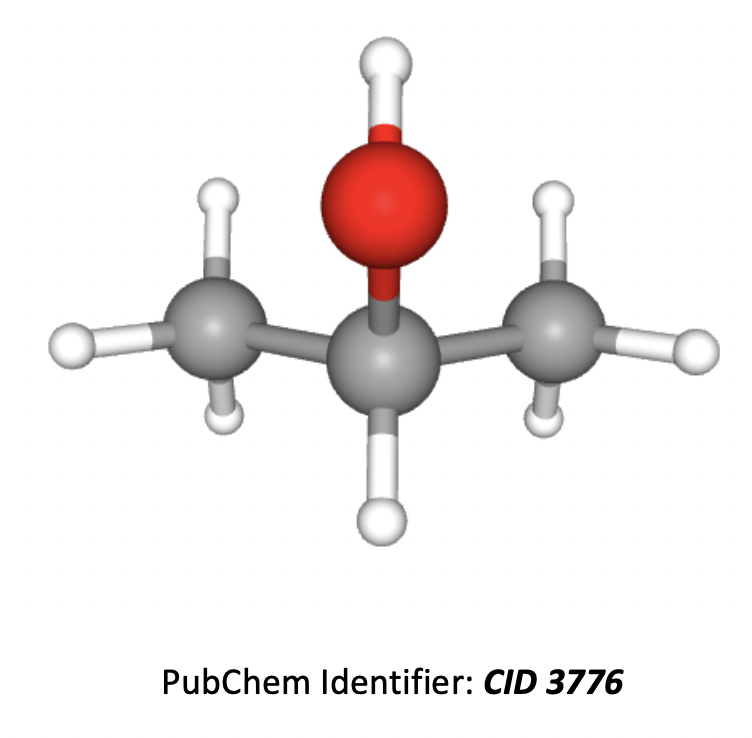
The hydrogens in the two CH3 groups on the ends of the molecule are all in the same environment (the molecule is symmetrical). So there are three hydrogen environments in total (not four).
The ratio of hydrogens in the environments is 1:1:6 (CH, OH and the two CH3 groups) so the integration trace will show a 1:1:6 ratio.
The H on the middle carbon will split the two CH3 (six hydrogen) peak into two (doublet), according to the n+1 rule. The two CH3 groups (6 hydrogens) will spilt the single C-H into a multiplet (theoretically into 7).
Remember that OH hydrogens tend not to split or be split. So the hydrogen on the OH will be a singlet peak.
Therefore 2 only is the correct answer.
How much of Measurement AHL (HL only) paper 1 questions have you understood?



 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn