 All matter is made up of particles (particle model). Since particles are constantly moving (kinetic theory), we should be able to explain all the properties of matter in terms of particle motion.
All matter is made up of particles (particle model). Since particles are constantly moving (kinetic theory), we should be able to explain all the properties of matter in terms of particle motion.
On this page, we will consider states of matter, forces and Brownian motion in relation to the particle model.
Key Concepts


Avogadro worked out that the number of particles in equal volumes of different gases at the same pressure and temperature must be the same.
Take a moment to consider the signficance of this statement. In the room where you are sitting, regardless of whether it contained air, pure oxygen, argon or chlorine, it would contain exactly the same number of particles. (Your lungs might notice though...)
This led to Avogadro's constant, defined on the Thermal concepts page.
\(N_A\) = 6.03 x 1023
When this number of particles are grouped together, the collective term is the mole (n). So, to calculate the number of moles present in a sample, divide the number of particles by Avogadro's constant:
\(n={N\over N_A}\)
How much of Particle model (kinetic theory) have you understood?


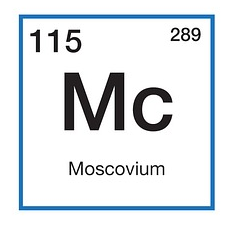
 Elements in the Periodic Table are displayed with a symbol (an uppercase letter, usually followed by a lowercase letter) and two numbers. The smaller atomic number represents the number of protons in the nucleus. The larger relative atomic mass number represents the sum of the protons and neutrons in the nucleus.
Elements in the Periodic Table are displayed with a symbol (an uppercase letter, usually followed by a lowercase letter) and two numbers. The smaller atomic number represents the number of protons in the nucleus. The larger relative atomic mass number represents the sum of the protons and neutrons in the nucleus. Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn