 This section introduces the classification of elements in the periodic table. The section has a small amount of content, but some key principles. The arrangement of an element in the periodic table allows us to predict its electron configuration (section 2.2); practice with the questions will help with understanding. Make sure you use the group numbering scheme from Group 1 to Group 18, as recommended by IUPAC, but also remember the 'old Roman numeral' group numbering system (using the Bohr model of the atom) which is so useful for Lewis diagrams.
This section introduces the classification of elements in the periodic table. The section has a small amount of content, but some key principles. The arrangement of an element in the periodic table allows us to predict its electron configuration (section 2.2); practice with the questions will help with understanding. Make sure you use the group numbering scheme from Group 1 to Group 18, as recommended by IUPAC, but also remember the 'old Roman numeral' group numbering system (using the Bohr model of the atom) which is so useful for Lewis diagrams.
Ensure you are confident using the terms below and learn the asterisked* definitions
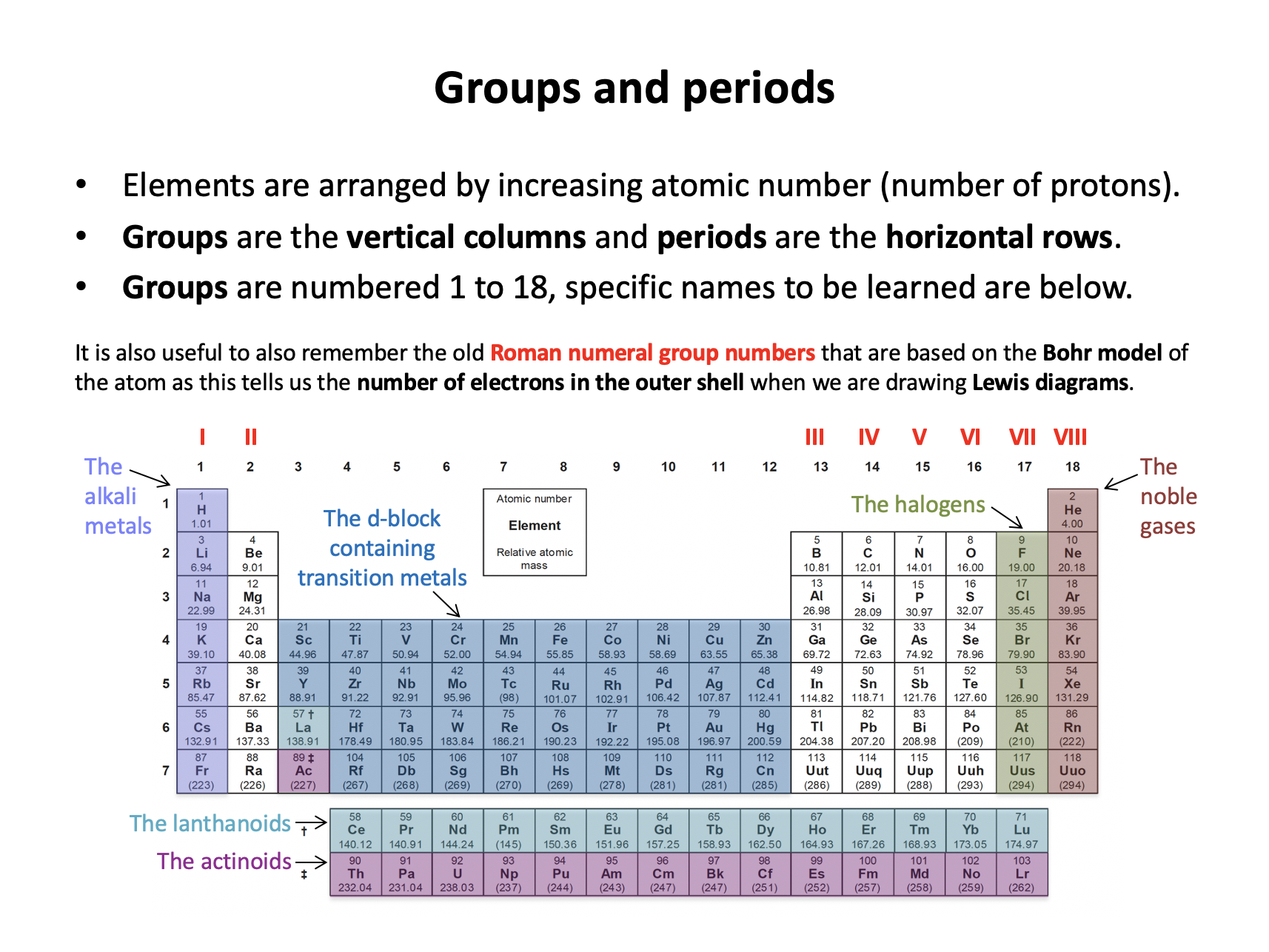
group*, period*, the alkali metals, the halogens, the noble gases, the d-block containing transition metals, the lanthanoids (lanthanides), the actinoids (actinides), metals, non-metals, metalloids.
What names do we give to groups 1 and 17 respectively?
Some group names need to be learned:
Alkali metals (group 1), Transition metals (d-block), Halogens (group 17), Noble gases (group 18), Lanthanoids (first row of f-block) and Actinoids (second row of f-block).
Thus Alkali metals and halogens is the correct answer.
Using the periodic table, in which block are the elements (atomic numbers in brackets) Promethium, Pm (61), Tungsten, W (74) and Bismuth, Bi (83) respectively?
Using a copy of the periodic table, which of these elements are lanthanoids (atomic numbers in brackets)?
1 Si (Z=14)
2 Nd (Z=60)
3 Cm (Z=96)
Lanthanoids are elements from Z=57 (La) to Z=71 (Lu). The first row of the f-block. The correct answer is therefore: 2 only
Using a copy of the periodic table, what is the correct electronic configuration for an atom of strontium, Sr (Z=38)?
Strontium is in the 5th period and is the second element of the s-block. Its outermost electron configuration is therefore 5s2.
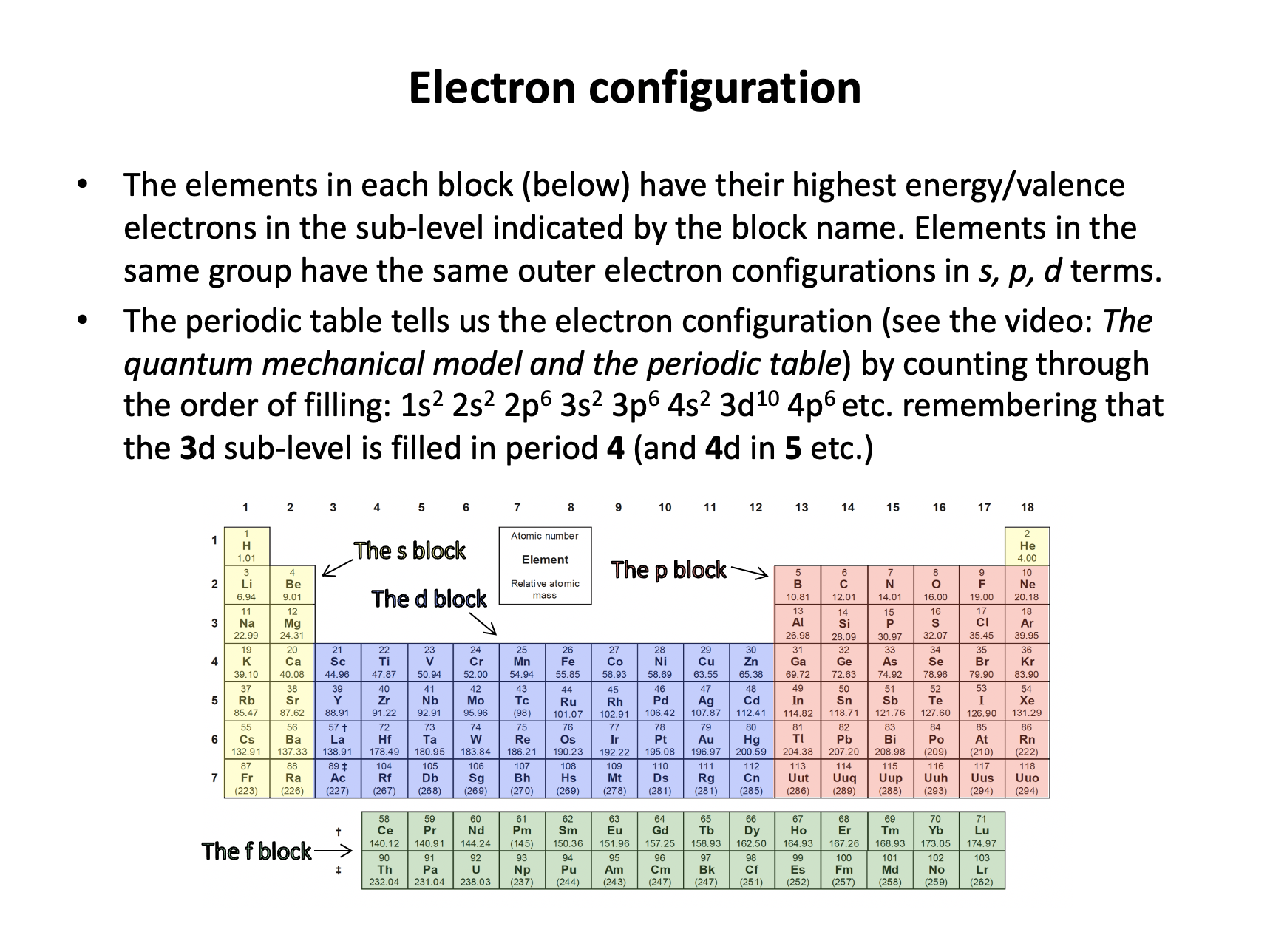
Filling it up in order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d etc. gives the correct answer:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2
Remember: The s-block is groups 1 and 2 (I and II); the d-block is groups 3-12; and the p-block is groups 13-18 (III to VIII). The period number corresponds to the energy level for the outermost electrons in the s and p blocks (one less for the d-block e.g. vanadium in period 4 has electrons 3d3 not 4d3).
Using a copy of the periodic table, what is the correct electronic configuration for an atom of tin, Sn (Z=50)?
Tin is in the 5th period and is the second element of the p-block. Its outermost electron configuration is therefore 5p2.
Filling it up in order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s etc. gives the correct answer:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p2
Remember: The s-block is groups 1 and 2 (I and II); the d-block is groups 3-12; and the p-block is groups 13-18 (III to VIII). The period number corresponds to the energy level for the outermost electrons in the s and p blocks (one less for the d-block e.g. vanadium in period 4 has electrons 3d3 not 4d3).
Using a copy of the periodic table, what is the correct electronic configuration for an atom of iron, Fe (Z=26)?
Iron is in the 4th period and is the sixth element of the s-block. Its outermost electron configuration is therefore 3d6 (Not 4d6 because 4s fills before 3d - see section 2.2 electron configuration).
Filling it up in order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p etc. gives the correct answer:
1s2 2s2 2p6 3s2 3p6 4s2 3d6
Remember: The s-block is groups 1 and 2 (I and II); the d-block is groups 3-12; and the p-block is groups 13-18 (III to VIII). The period number corresponds to the energy level for the outermost electrons in the s and p blocks (one less for the d-block e.g. vanadium in period 4 has electrons 3d3 not 4d3).
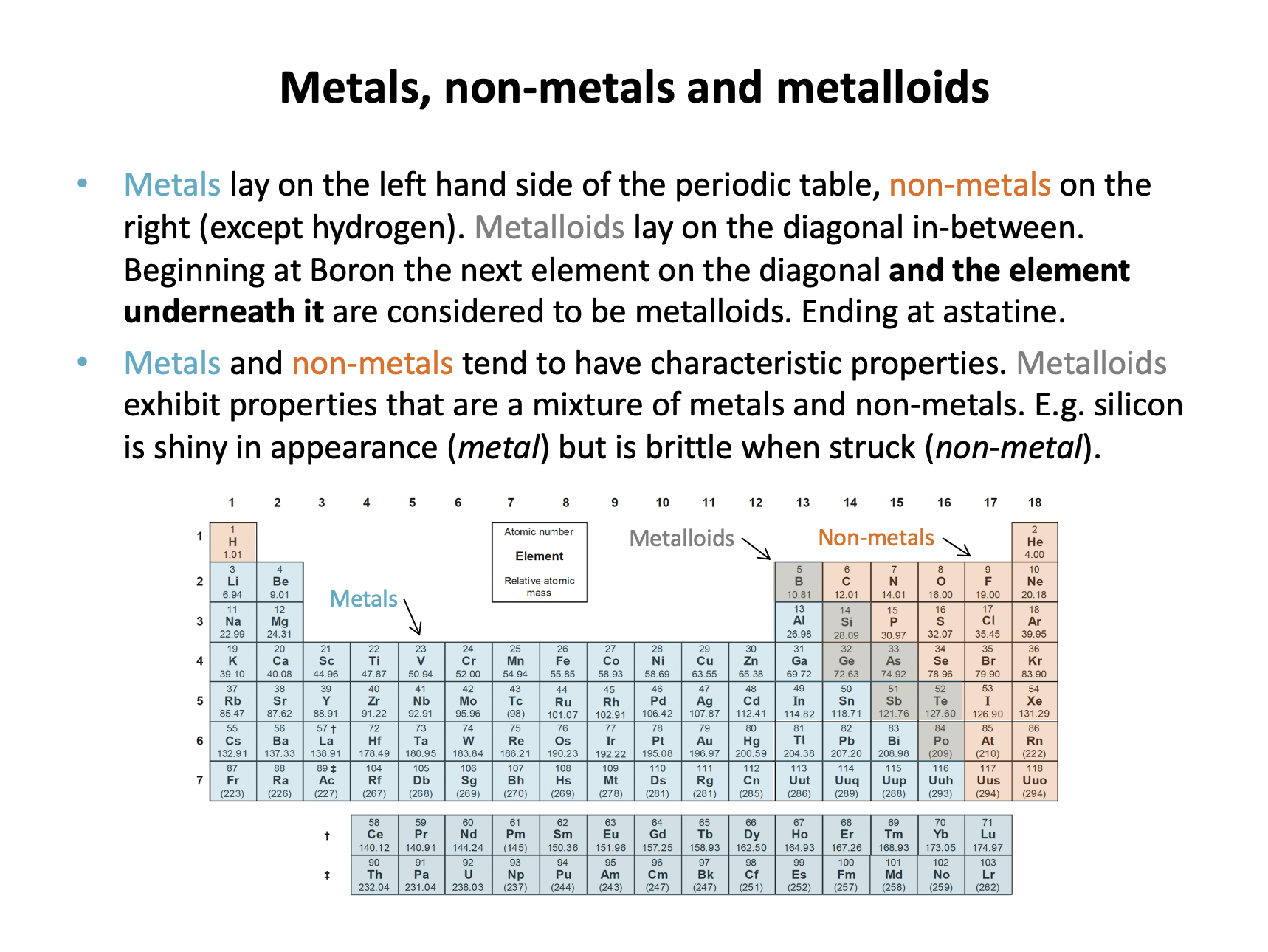
Using a copy of the periodic table, which of these elements are metalloids?
1 Germanium
2 Boron
3 Tin
Metalloids lay along the diagonal line between metals and non-metals, beginning with Boron. It is worth remembering that after Boron, on the diagonal the next element and the element underneath it are considered to be metalloids. Ending at astatine, which is considered to be a halogen (and therefore a non-metal).
Thus 1 and 2 only is the correct answer.
Paper 1
Core (SL&HL): Periodicity core (SL and HL) paper 1 questions
AHL (HL only): Periodicity AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Periodicity core (SL & HL) paper 2 questions
AHL (HL only): Periodicity AHL (HL only) paper 2 questions
How much of Periodic table have you understood?







 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn