 This is a relatively short section of the course. Coloured compounds arise in d-block elements due to the splitting in energy of the d sub-levels when ligands surround the central metal ion. Understanding that idea is the key to this topic. Everything else is relatively straightforward, but make sure you know your First-row d-block elements (13.1) content before you begin your revision here.
This is a relatively short section of the course. Coloured compounds arise in d-block elements due to the splitting in energy of the d sub-levels when ligands surround the central metal ion. Understanding that idea is the key to this topic. Everything else is relatively straightforward, but make sure you know your First-row d-block elements (13.1) content before you begin your revision here.
Ensure you are confident using the terms below and learn the asterisked* definitions
degenerate, energy gap (ΔE), complementary colour, strength of a ligand
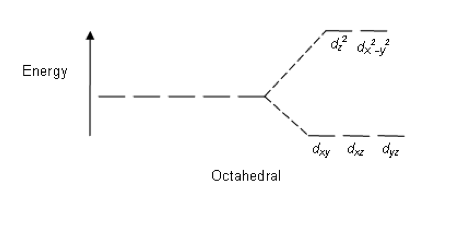
When ligands approach a first row transition metal ion in an octahedral complex, how do the five 3d-orbitals split in energy?
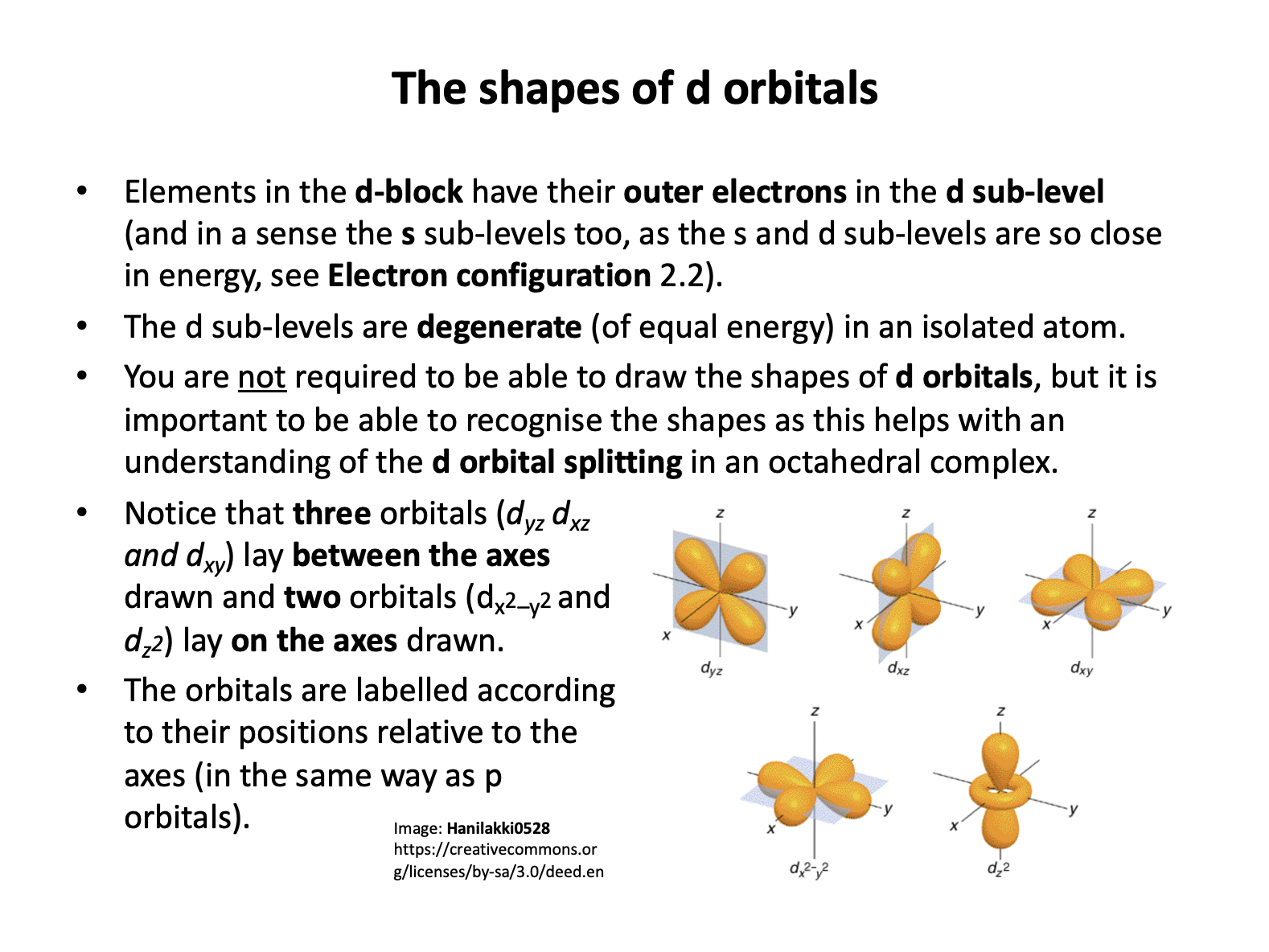
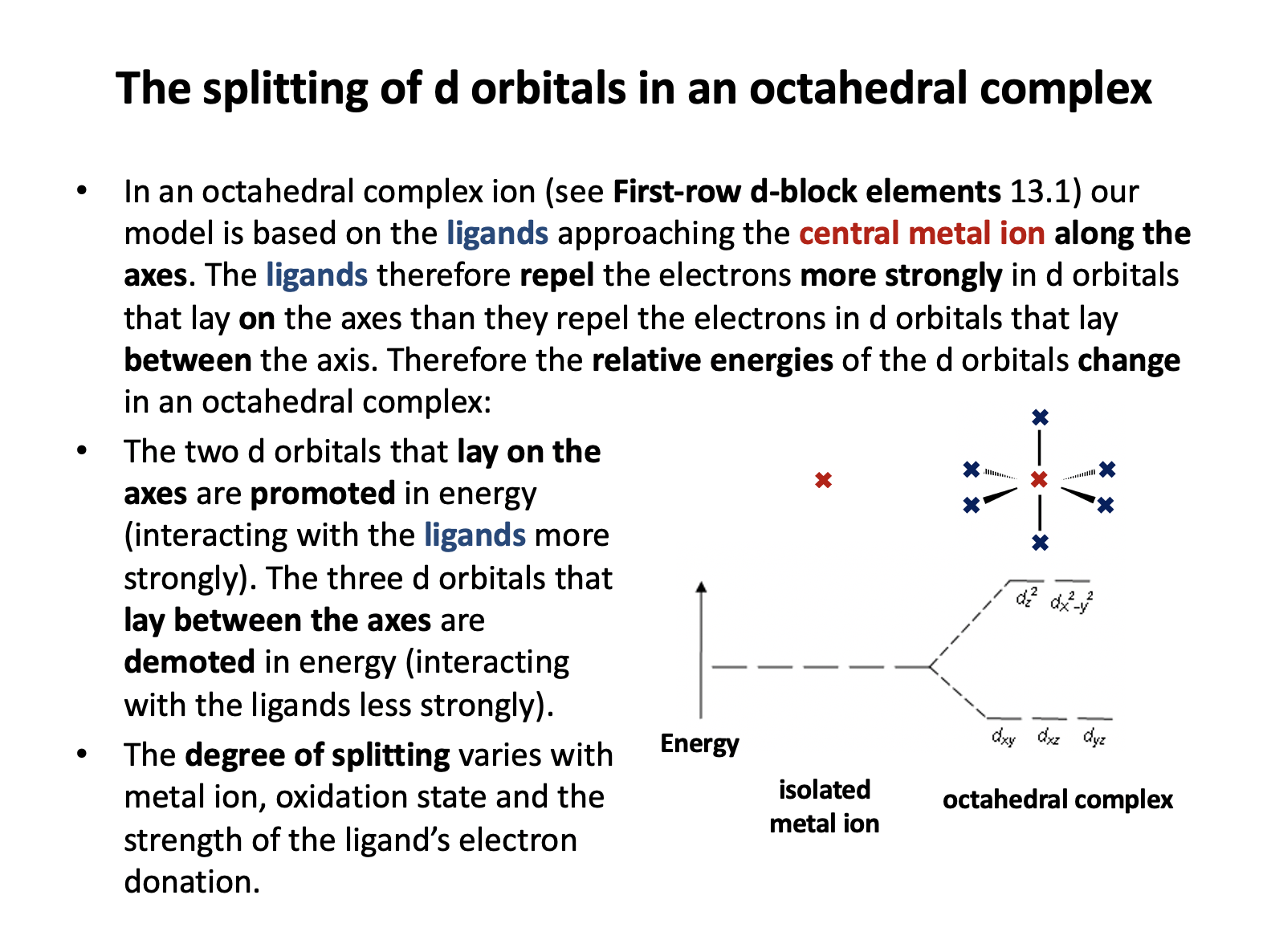
The approach of the octahedral ligands interacts more strongly with d-orbitals that lay on the axes than d-orbitals that lay between the axes, so the dz2 and dx2−y2 (that both lay on the axes) increase in energy and the other orbitals (that lay between the axes) decrease in energy.
Thus 'two up; three down' is the correct answer.
In transition metal octahedral complex ions, the d-orbitals split into two sets of orbitals at different energies. Which explanantion best explains why these complexes are coloured?
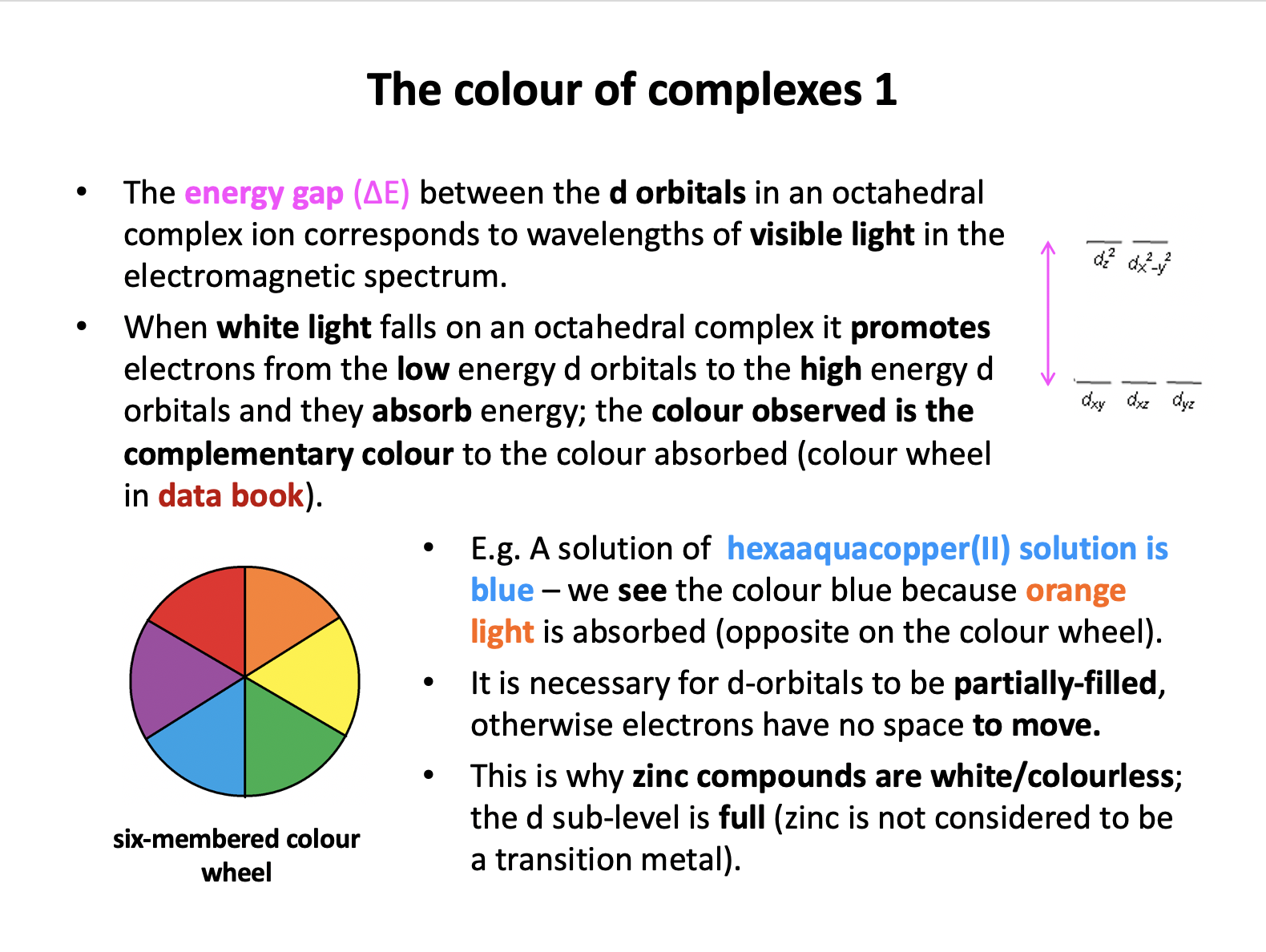
Transition metal compounds are coloured because the energy gap between the two sets of d-orbitals lays in the visible region of the spectrum. When visible light falls on the compound some of it is absorbed and promotes electrons from low to high energy d-orbitals; the colour observed is the complementary colour to the colour/frequency of light absorbed (colour wheel in data book) e.g. copper sulfate solution is blue – orange light is absorbed.
Thus 'Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour of light absorbed' is the correct answer.
Which of the following contributes to the coloured nature of many transition metal compounds?
1: The splitting of the d-orbitals in energy.
2: The d-orbitals are partially filled with electrons.
3: The energy gap between the d-orbitals that have been split lays in the visible region of the spectrum.
Transition metal compounds are coloured because:
- Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies
- The energy gap corresponds to wavelengths of visible light.
- Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed (colour wheel in data book) e.g. copper sulfate solution is blue – orange light is absorbed.
- It is necessary for d-orbitals to be partially-filled or electrons have no space to move.
Thus 1,2 and 3 is the correct answer.
Why are octahedral complexes of zinc not coloured?
Transition metal compounds are coloured because:
- Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies
- The energy gap corresponds to wavelengths of visible light.
- Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed (colour wheel in data book) e.g. copper sulfate solution is blue – orange light is absorbed.
- It is necessary for d-orbitals to be partially-filled or electrons have no space to move.
Zinc forms only Zn2+ ions that are stable - with electronic configuration [Ar] 4s0 3d10
In an octahedral complex of zinc the d-orbitals do split, and the energy gap does lie in the visible region, but as the orbitals are all full (the 3d-sub level is full) there is no space for electrons to move, so the complexes are colourless.
Thus 'Zinc has a 3d sub-level that is full of electrons.' is the correct answer.
If a transition metal complex ion absorbs strongly in the green region of the electromagnetic spectrum, what colour is the complex likely to be?
Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies and the energy gap corresponds to wavelengths of visible light.
Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed (colour wheel in data book)
On the colour wheel, 'red' is opposite green and is thus the correct answer.
If a transition metal complex ion absorbs strongly at a peak of 580nm in the region of the electromagnetic spectrum, what colour is the complex likely to be?
Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies and the energy gap corresponds to wavelengths of visible light.
Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed (colour wheel in data book)
On the colour wheel 580nm corresponds to yellow light and opposite this the colour is 'violet' which is thus the correct answer.
Which of the following ligands is likely to cause the greatest energy difference between the two sets of d-orbtials in an octahedral transition metal complex ion?
Using the spectrochemical series in the data book (below), it can be seen that of the ligands given ammonia causes the greatest splitting. Thus, NH3 is the correct answer.
![]()
A complex of copper (II) is octahedral and bright blue in colour.
If ligand exchange takes place by addition (in excess) of an alternative ligand, the complex changes colour. The new complex is octahedral and dark purple-blue in colour.
What has happened to the energy gap between the the two sets of 3d-orbitals in going from the first to the second copper complex?
Ligands in an octahedral complex cause splitting of d-orbitals into two sets at different energies and the energy gap corresponds to wavelengths of visible light.
Absorption of visible light promotes electrons from low to high energy d-orbitals and the colour observed is the complementary colour to the colour/frequency of light absorbed.
Using the colour wheel in data book: The colour of the first complex is blue suggesting an absorbance in the orange region (585-647nm) and the second complex is purple-blue suggesting that the absorbance has moved to a lower wavelength; towards the yellow region (575-585nm). Lower wavelength means greater frequency/energy, so the gap between the two sets of 3d-orbitals must have increased.
'The energy gap has increased' is thus the correct answer.
Paper 1
Core (SL&HL): Periodicity core (SL and HL) paper 1 questions
AHL (HL only): Periodicity AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Periodicity core (SL & HL) paper 2 questions
AHL (HL only): Periodicity AHL (HL only) paper 2 questions
How much of Coloured complexes have you understood?









 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn