 This section of the course is not in itself too conceptually challenging, but there are lots of links to other parts of the course. Ensure that you are confident with electronic structure (quantum mechanical model of the atom), know about dative bonds and shapes of molecules, and understand Lewis acids and bases. Then it is a matter of learning the content here.
This section of the course is not in itself too conceptually challenging, but there are lots of links to other parts of the course. Ensure that you are confident with electronic structure (quantum mechanical model of the atom), know about dative bonds and shapes of molecules, and understand Lewis acids and bases. Then it is a matter of learning the content here.
Ensure you are confident using the terms below and learn the asterisked* definitions
A transition element*, A ligand*, A dative (coordinate) bond*, A complex ion*, Coordination number*, Monodentate ligand, Polydentate ligand, Paramagnetism, Ferromagnetism, Diamagnetism
Which of the following describes the typical properties of a transition metal?
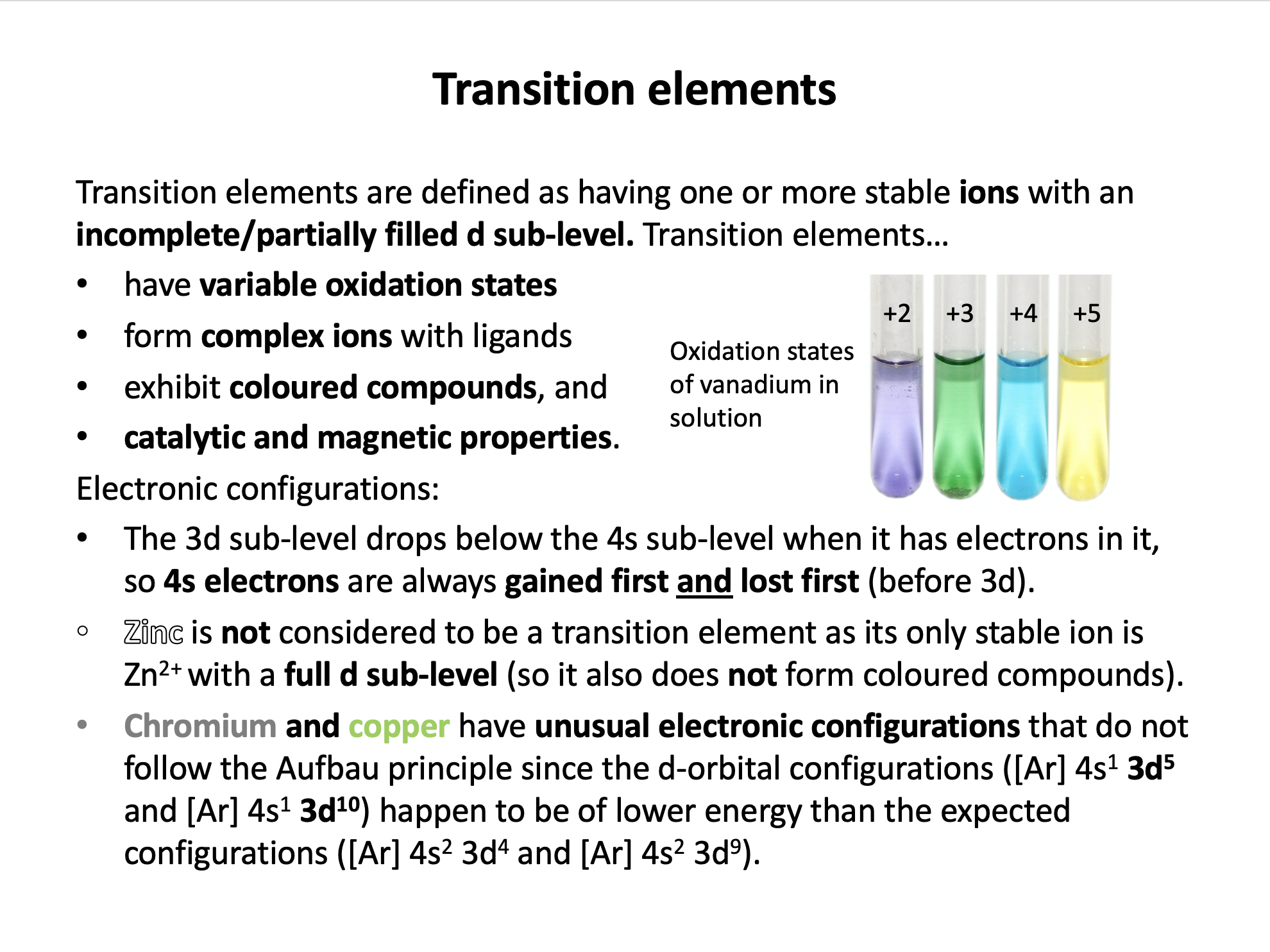
1: They have variable oxidation states.
2: They display catalytic properties.
3: They are Lewis acids.
Transition metals have variable oxidation states, form complex ions with ligands (ligands donate electron pairs into the metal centre, so the transition metal is behaving as a Lewis acid), have coloured compounds, and display catalytic and magnetic properties. Thus 1,2 and 3 is the correct answer.
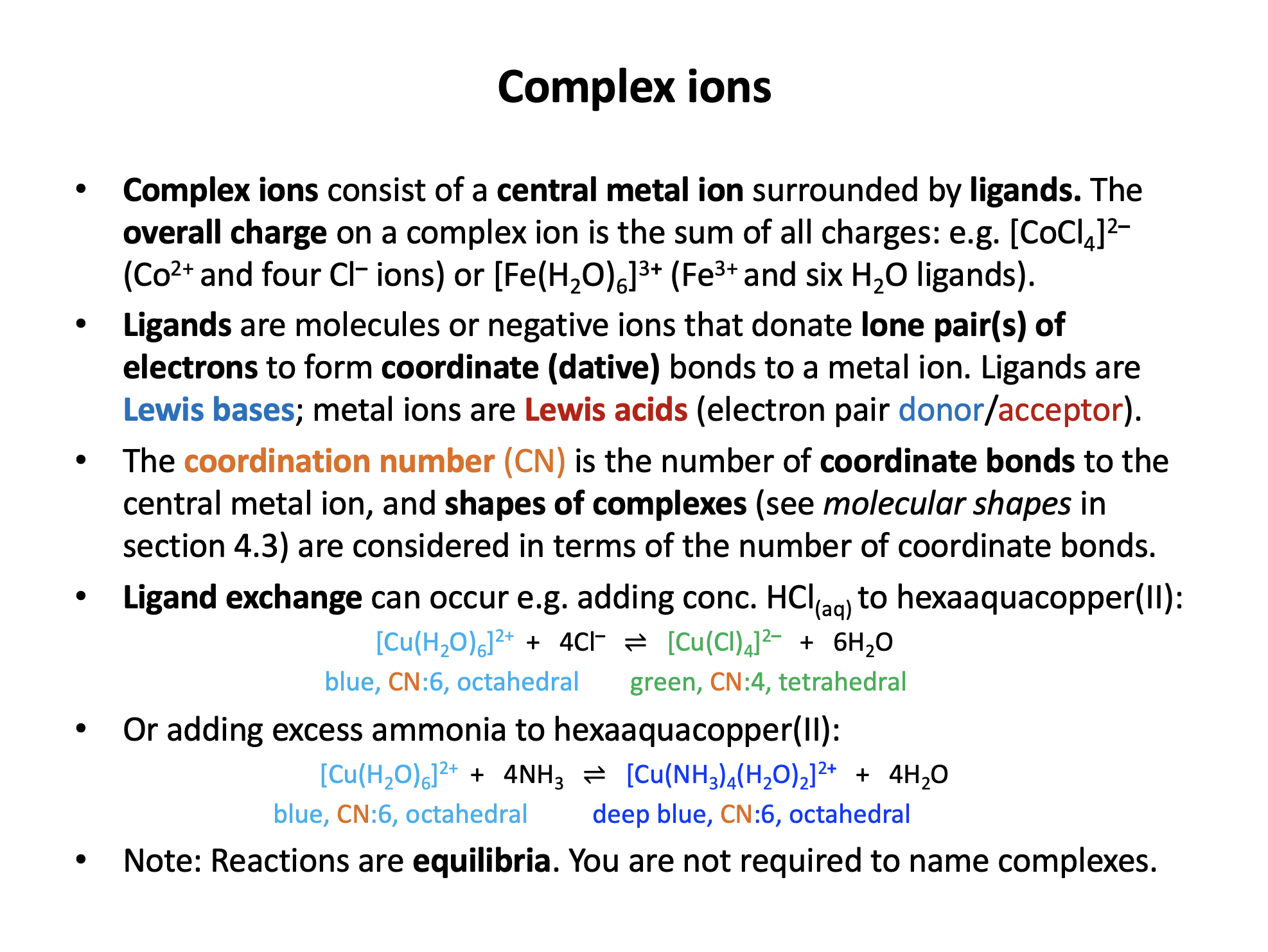
Which of the following cannot act as a ligand?
A ligand must have a lone pair of electrons that can be donated to a metal centre to form a coordinate (dative) bond. All of the species written have a lone pair except for the ammonium ion (NH4+) which does not, and is therefore the correct answer.
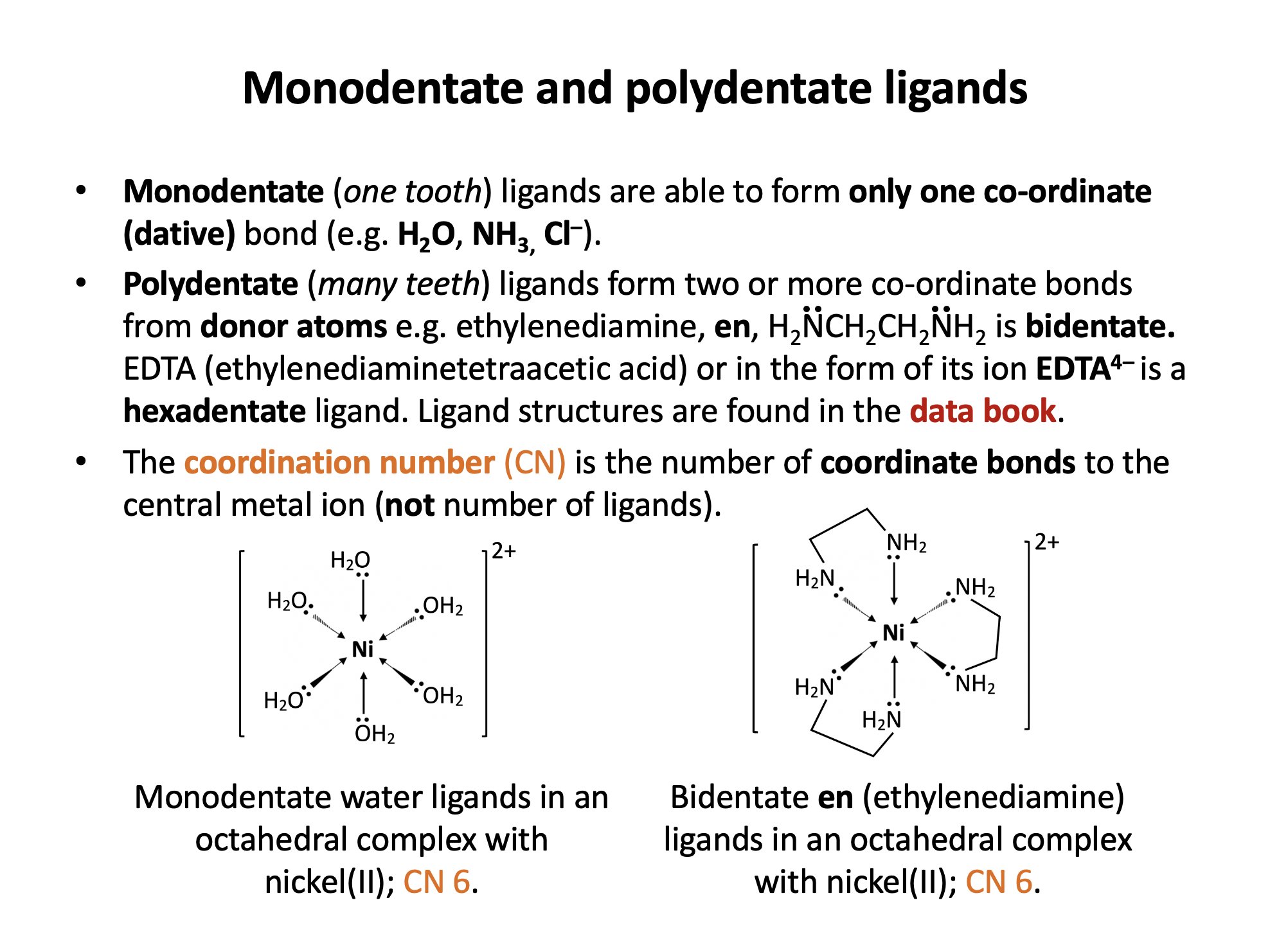
A complex ion has the formula [Ni(en)3]2+.
What is the coordination number of the nickel ion, and what is the denticity (number of coordinate bonds that a single ligand can form to a metal centre) of the 'en' ligand?
The 'en' ligand is 1,2-ethanediamine and its structure is shown in the data book (section 16 Ligands). As can be seen from the structure it has two amine groups capable of donating a pair of electrons and it is therefore bidentate.
If each 'en' forms two coordinate bonds to the nickel ion, and there are three ligands, there are a total of six coordinate bonds into the nickel centre - the coordination number is 6.
'6 ; bidentate' is the correct answer.
Which of the following d-block metal compounds is not coloured?
Most transition metal compounds are coloured, but Zn is not considered to be a transition metal (despite being in the d-block) because it does not form ions with incomplete d-orbitals (It is only stable as Zn2+ in which it has lost both 4s electrons and has a complete 3d sub-level). Without incomplete d-orbitals in the metal d-block compounds cannot be coloured, so Zinc compounds, like ZnO, are not coloured. ZnO is the correct answer.
ZnO is a white solid, CuCO3 is a blue-green solid, Fe2O3 is a red-brown solid, [Fe(H2O)5SCN]2+ is a blood-red solution.
What is the overall (missing) charge on the copper (II) complex ion: [CuCl4] ?
The charge on the copper ion is 2+ (given by the Roman numerals, II). Each chloride ligand (four are found in the complex) has a charge of 1−, so the overall charge will be 2+ + (4×1−) = 2−.
Charge is always expressed as number then sign, that is 2−.
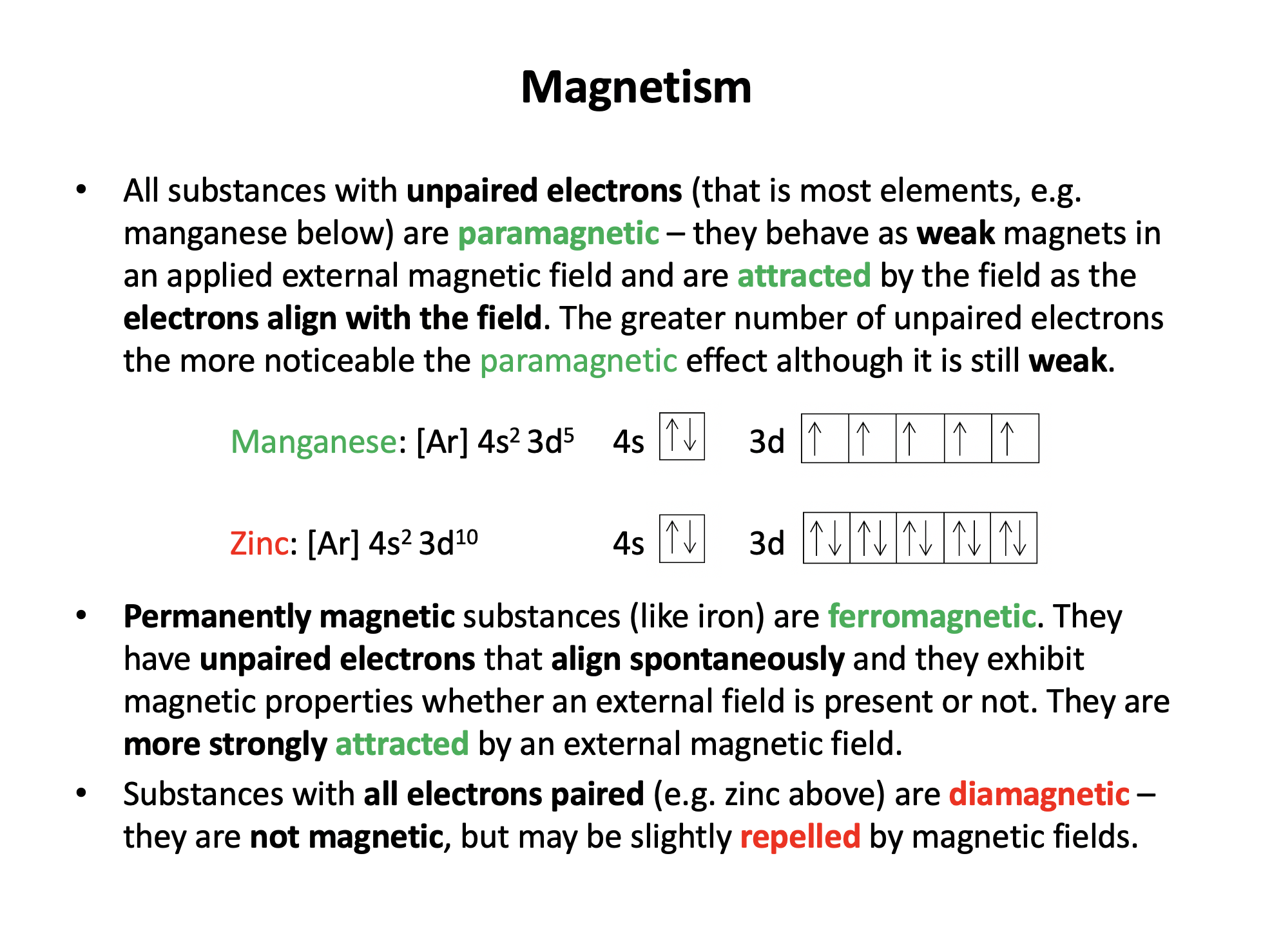
Some magnetic substances behave as if they are weakly magnetic when an external magnetic field is applied.
Which type of magnetism does this sentence descibe?
Ferromagnetic substances, like iron, are permanently magnetic.
Paramagnetic substances have unpaired electrons, and behave as if they are weakly magnetic when an external magnetic field is applied.
Diamagnetic substances have all electrons paired, are not magnetic at all, and may be weakly repelled by an external magnetic field.
Thus paramagnetism is the correct answer here.
Which of the following contributes to the ability of transition metals to show catalytic activity?
1: The relatively high melting points.
2: The ability to form variable oxidation states.
3: The ability to adsorb substances onto their surface.
Transition metals can often act as catalysts because of their ability to switch between oxidation states, and because substances can easily adsorb onto the surface of a metal.
There is no evidence that the relatively high melting points of transition metals contributes to their catalytic activity.
Thus '2 and 3 only' is the correct answer.
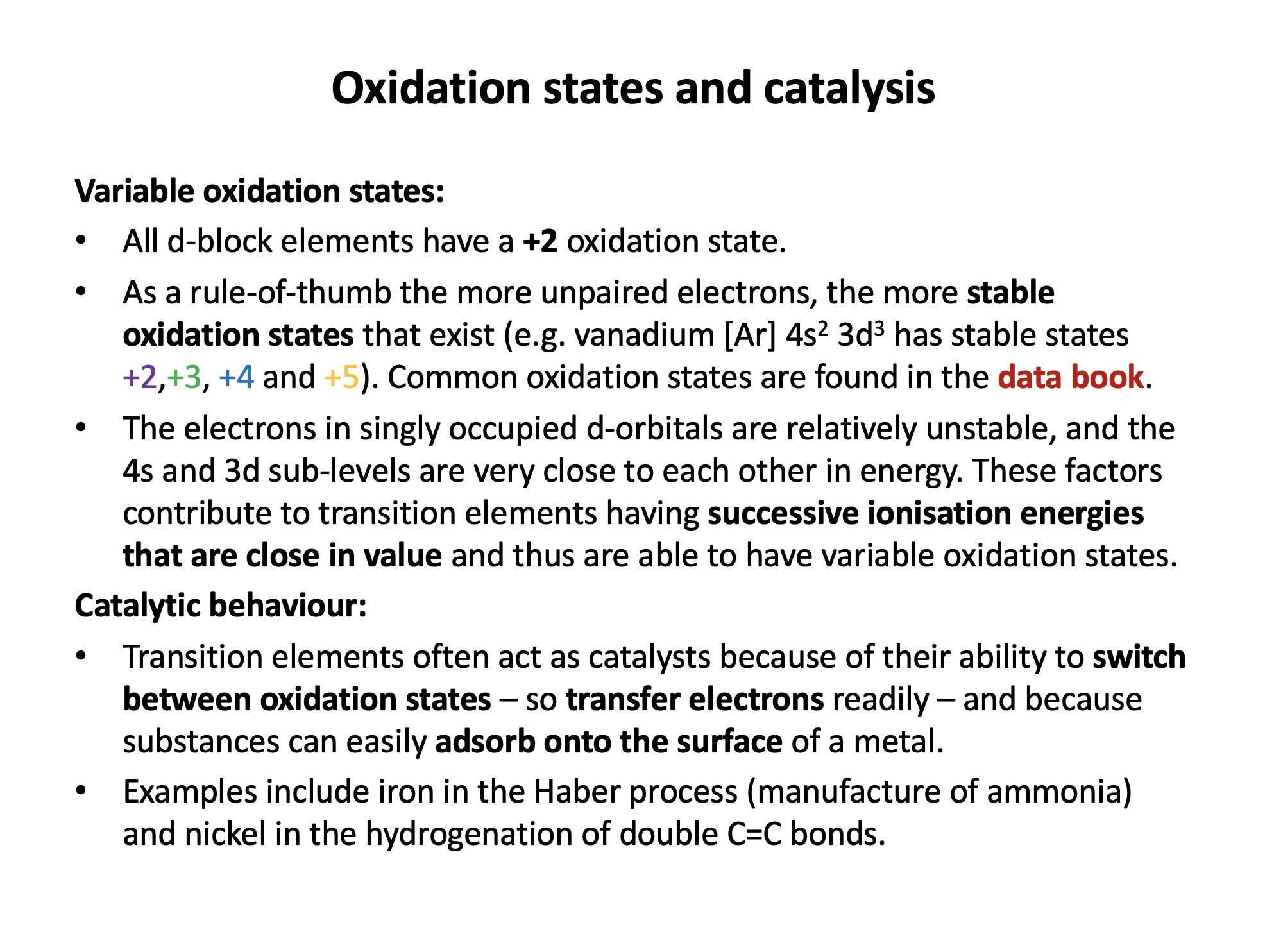
Which of the following contributes to the ability of transition metals to form variable oxidation states?
1: The relatively (compared to group 1 and 2 metals) low first ionisation energies.
2: The presence of singly-occupied d-orbitals.
3: The closeness in energy of the s and d sub-levels.
Transition metals do not have low first ionisation energies when compared to the main group metals.
The electrons in singly occupied d-orbitals are relatively unstable, and the s and d sub-levels are very close to each other in energy (remember that 's-electrons' are gained first and lost first). These factors contribute to transtion metals having successive ionisation energies that do not have 'big jumps' and thus being able to have variable oxidation states; thus 2 and 3 only is the correct answer.
Which of the following are paramagnetic?
1: Oxygen atoms
2: Zinc atoms
3: Vanadium atoms
Paramagnetic substances have unpaired electrons, and behave as if they are weakly magnetic when an external magnetic field is applied.
Diamagnetic substances have all electrons paired, are not magnetic at all, and may be weakly repelled by an external magnetic field.
Oxygen and vanadium atoms both have unpaired electrons in their electronic configurations (1s2 2s2 2p4 and 1s2 2s2 2p6 3s2 3p6 4s2 3d3 respectively), but zinc atoms do not (1s2 2s2 2p6 3s2 3p6 4s2 3d10). Remember that all orbitals in a given sub-level are filled singly first, before electrons pair-up.
Thus '1 and 3 only' is the correct answer.
Paper 1
Core (SL&HL): Periodicity core (SL and HL) paper 1 questions
AHL (HL only): Periodicity AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Periodicity core (SL & HL) paper 2 questions
AHL (HL only): Periodicity AHL (HL only) paper 2 questions
How much of First-row d-block elements have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn