 You are likely to have met much of this topic in your previous chemistry studies. Confidence with your recall of the collision theory and the effect of temperature, surface area and concentration upon rate of reaction is critical. It is also important to understanding of the Maxwell-Boltzmann distribution and be able to draw it (so practise this)!
You are likely to have met much of this topic in your previous chemistry studies. Confidence with your recall of the collision theory and the effect of temperature, surface area and concentration upon rate of reaction is critical. It is also important to understanding of the Maxwell-Boltzmann distribution and be able to draw it (so practise this)!
Ensure you are confident using the terms below and learn the asterisked* definitions
rate of reaction, activation energy*, catalyst*, successful collision, Maxwell-Boltzmann distribution, energy profile (energy level diagram)
Which of the following is an/are important consideration/s in collision theory when determining the rate of a chemical reaction?
1: The frequency of collisions
2: The energy of collisions
3: The orientation of collisions
Collision theory states that particles must collide with the correct orientation and with sufficient energy to have a sucessful collision (to overcome the activation energy) and thus react. However, the frequency of collisions will also be important in determining the rate of reaction, since a greater number of collisions per unit time will result in a greater number of successful collisions per unit time. Thus '1,2 and 3' is the correct answer.
Which of the following is not likely to be a unit of rate of reaction?
Rate of reaction can be measured in any unit of quantity per unit time. All the answers indicate a quantity changing with time (remember that mol dm–3 means moles per cubic decimetre and is a unit of concentration and min–1 means per minute) except 'g mol–1' which means grams per mole (the unit of molar mass) and is thus the correct answer.
Which of the following might be followed in an appropropriate chemical reaction when attempting to measure the rate of reaction?
1: The change in colour of reactants
2: The change in volume of products
3: The change in mass of reactants
'1, 2 and 3' is the correct answer because all these quantities can be measured to follow the rate in an appropriate chemical reaction. e.g. colour of bromine disappearing; volume of hydrogen gas produced; mass decrease as calcium carbonate reacts with acid (and carbon dioxide is given off).
Which of the following is the best definition of activation energy?
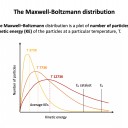
The correct (best) answer is 'The minimum energy that colliding particles need in order to react.' This is a definition that must be learned. The other answers are incorrect, although activation energy is required to 'break bonds' in reactants (but not necessarilly all bonds). The average kinetic energy of particles is often marked on a Maxwell-Boltzmann curve, and the enthalpy difference between reactants and products is part of the Energetics topic but neither are directly linked to activation energy.
Which of the following is/are true with respect to the action of a catalyst on a chemical reaction?
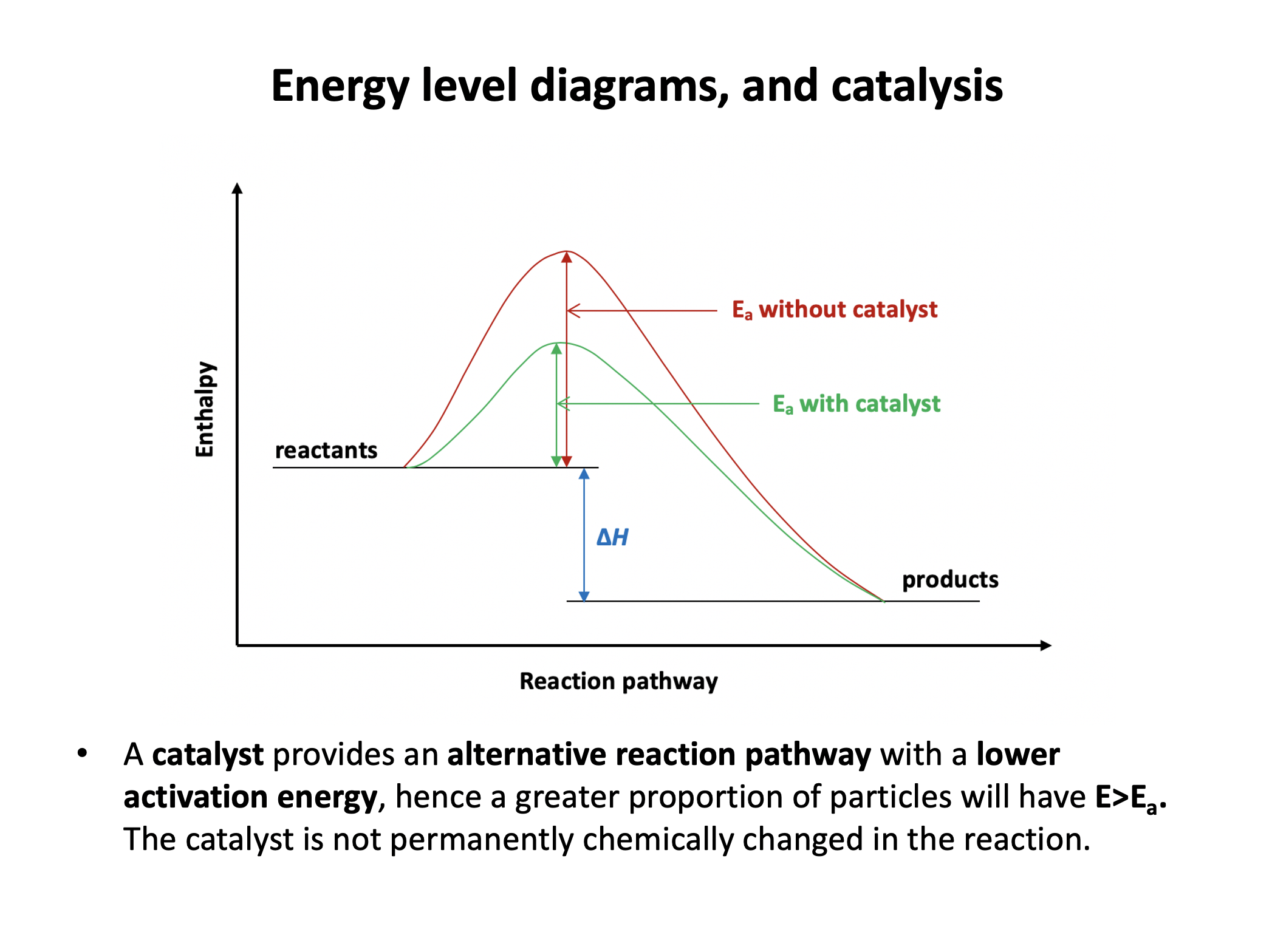
1: The catalyst lowers the activation energy
2: The catalyst is not permanently chemically changed during the reaction
3: The catalyst increases the energy of the particle collisions
A catalyst increases the rate of a chemical reaction by causing the reaction to follow a different reaction pathway of lower activation energy, and is not permanently chemically changed during the reaction. The catalyst does not otherwise affect the energy of the particles (or the position of equilibrium) - it does not increase the collision energy or collision frequency. Thus '1 and 2 only' is the correct answer.
Which of the following is the best explanation for why increasing temperature increases rate of reaction?
All these answers are correct statements, but 'Particles move faster, collide more often and collide with greater energy' is the correct (best) answer since it covers all the important points: Increasing temperature causes particles to have greater kinetic energy (move faster), increases the collision frequency (collide more often) and also causes collisions to have greater energy (because the particles are striking each other at higher speeds). This results in a greater proportion of collisions that can overcome the activation energy per unit time, and thus a greater rate of reaction.
Which of the following is the correct explanation for why increasing surface area increases rate of reaction?
Increasing surface area does not increase the energy of the particles or of the collisions, nor does it alter the activation energy to allow particles of lower energy to react. So the correct answer is 'A greater number of particles are exposed so there are a greater number of collisions per unit time.' Increasing surface area increases the collision frequency (particles collide more often). This results in a greater number of successful collisions per unit time, and thus a greater rate of reaction.
Which word is missing from the sentence below?
Increasing the .................................. may increase the rate of a chemical reaction because having a greater number of particles in the same volume increases the collision frequency of the particles so there will be more successful collisions per second.
The correct answer is 'concentration'. Increasing volume will lower concentration and may have the opposite effect (lower rate). Increasing temperature will increase the rate, but will not increase the number of particles in the same volume. Increasing activation energy would decrease the rate as fewer particles would have the minimum energy needed to react.
For a chemical reaction, which of the differences in energy below represents the activation energy of the reverse reaction?
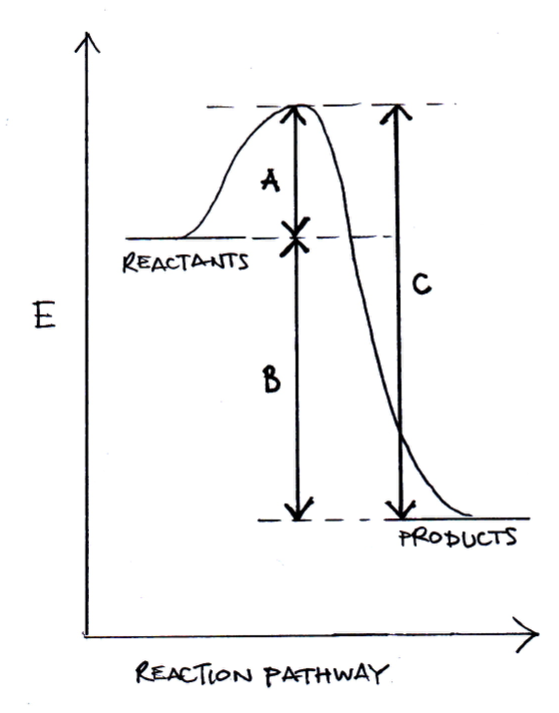
C is the activation energy of the reverse reaction and is the correct answer. A is the activation energy of the forward reaction; B is the difference in enthalpy between reactants and products; C minus B would be equal to A (activation energy in the forward direction).
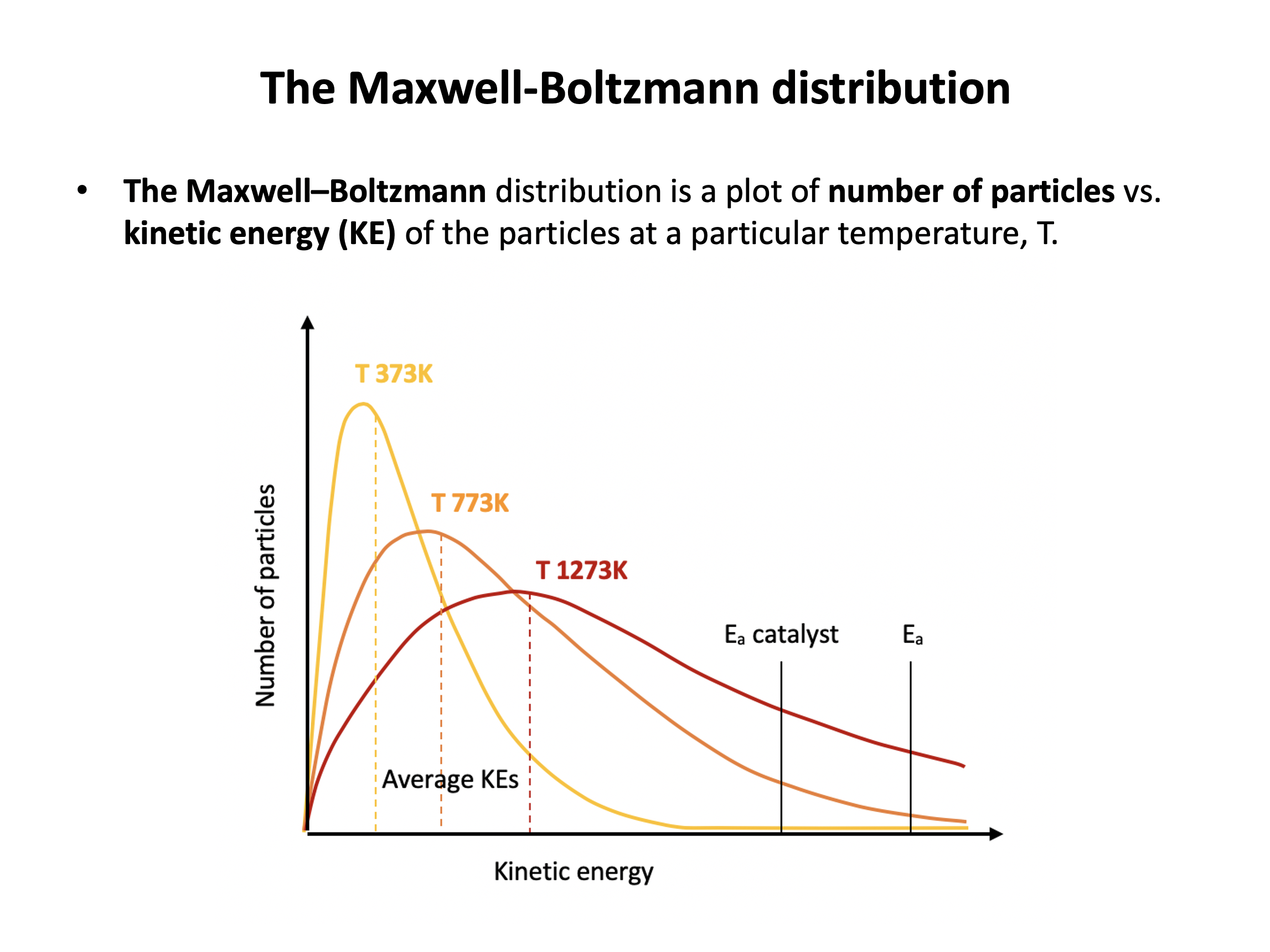
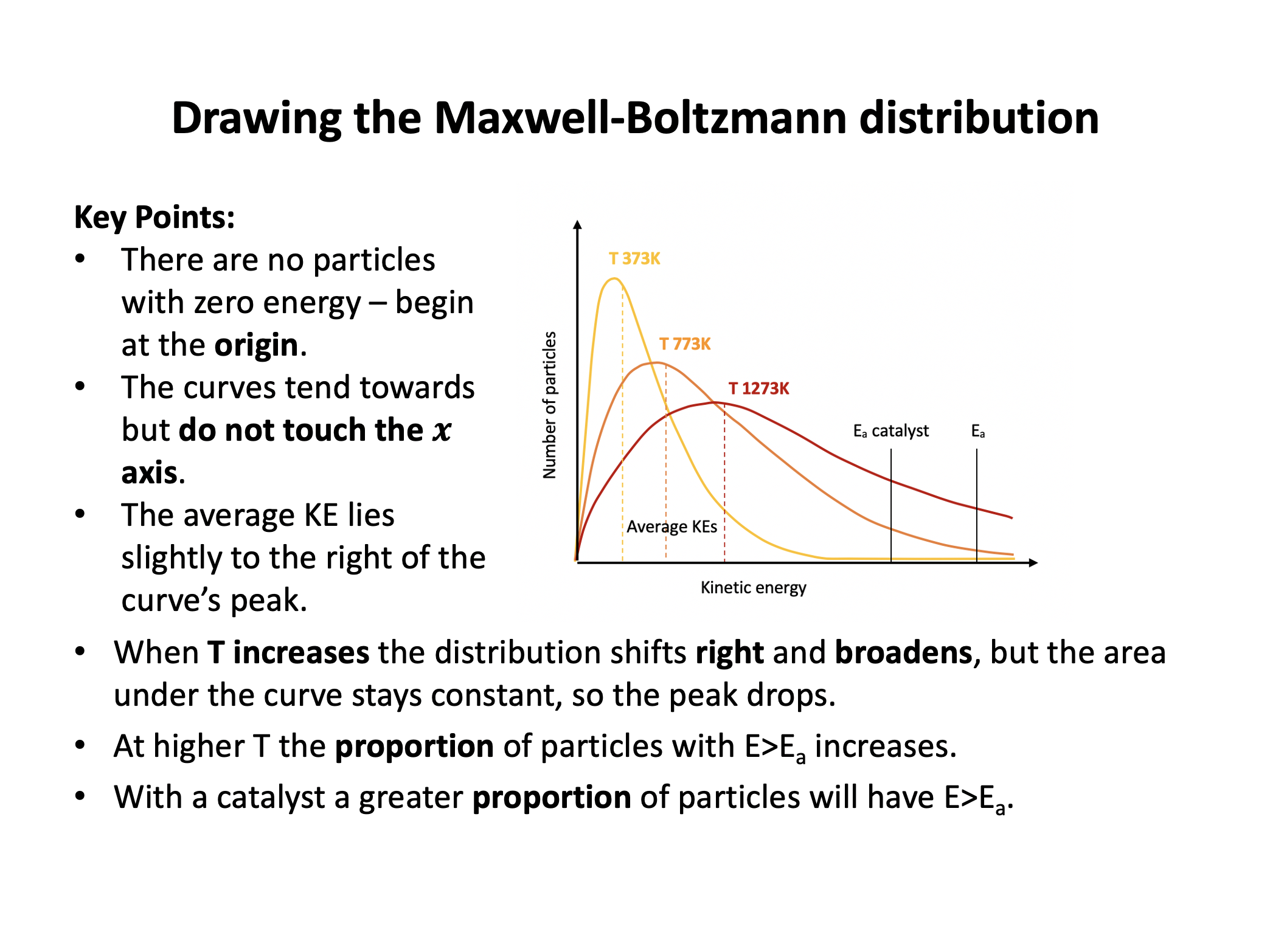
The diagram below represents a Maxwell-Boltzmann energy distribution.
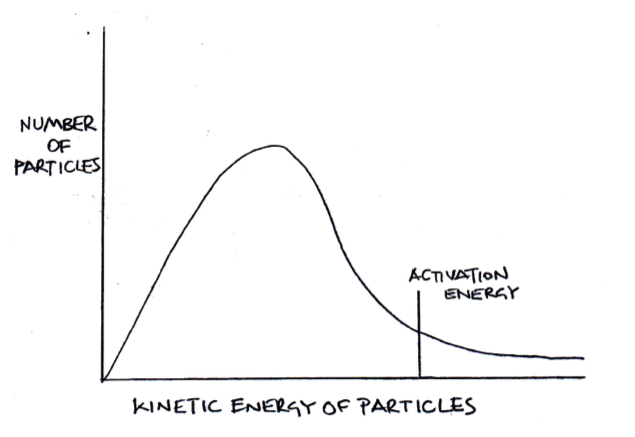
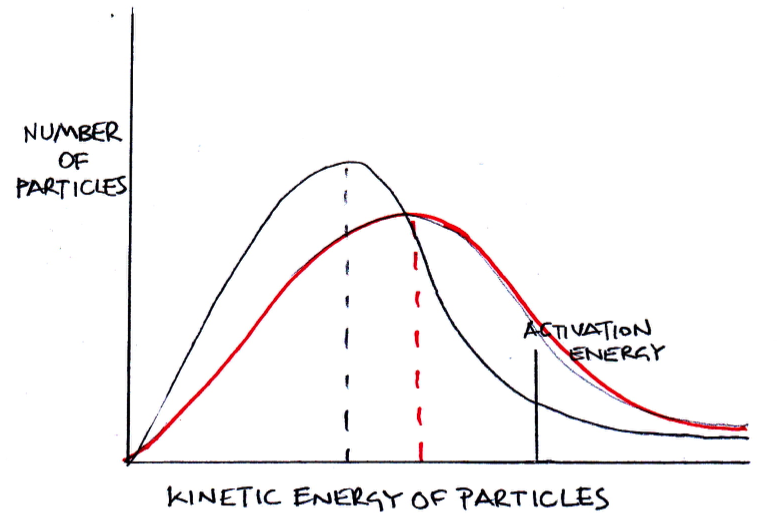
If the temperature is increased, which of the following correctly describe how the distribution should be redrawn?
1: The distribution should flatten out
2: The peak of the distribution should drop lower
3: The peak of the distribution should move to the right
If the temperature is increased particles will gain kinetic energy, but the number of particles will remain the same. Thus all three (1,2 and 3) correctly describe how the distribtuion should be redrawn as exemplified in red here:
Paper 1
Core (SL&HL): Kinetics core (SL and HL) paper 1 questions
AHL (HL only): Kinetics AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Kinetics core (SL & HL) paper 2 questions
AHL (HL only): Kinetics AHL (HL only) paper 2 questions
How much of Collision theory and rates of reaction have you understood?









 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn