 This section is more straightforward than it at first seems. The important thing is to be comfortable in identifying the order of reaction of a reactant or catalyst from data given, whether that is in the form of a graph or numerical data. Understanding the relationship between the rate equation and the reaction mechanism is the most challenging part of this topic - so focus on that last, once you are confident with everything else.
This section is more straightforward than it at first seems. The important thing is to be comfortable in identifying the order of reaction of a reactant or catalyst from data given, whether that is in the form of a graph or numerical data. Understanding the relationship between the rate equation and the reaction mechanism is the most challenging part of this topic - so focus on that last, once you are confident with everything else.
Ensure you are confident using the terms below and learn the asterisked* definitions
rate determining step*, molecularity*, order of reaction*, reaction mechanism, rate constant, rate equation, intermediate, transition state
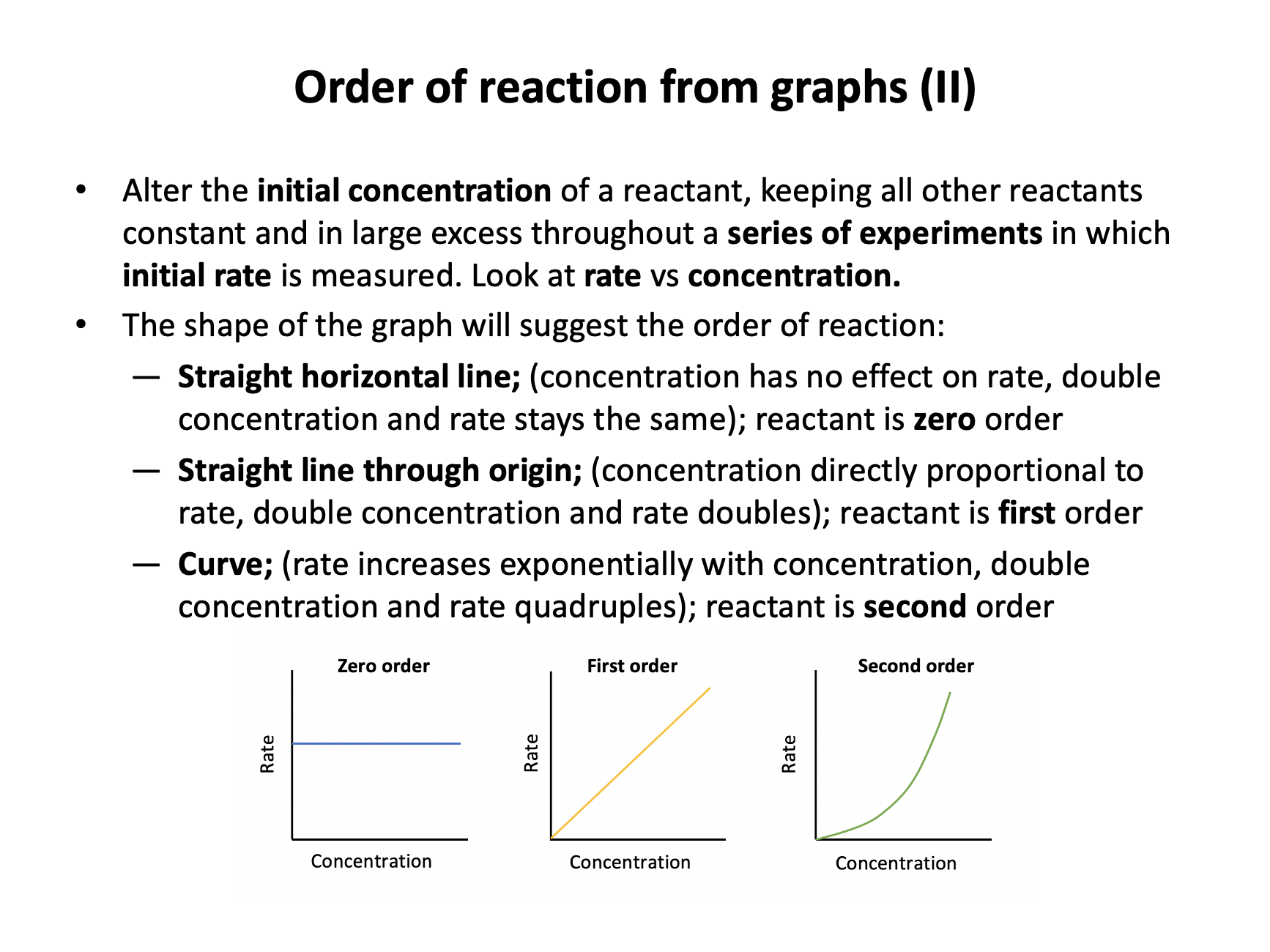
Study the following sketches of concentration vs rate graphs for three reactants:
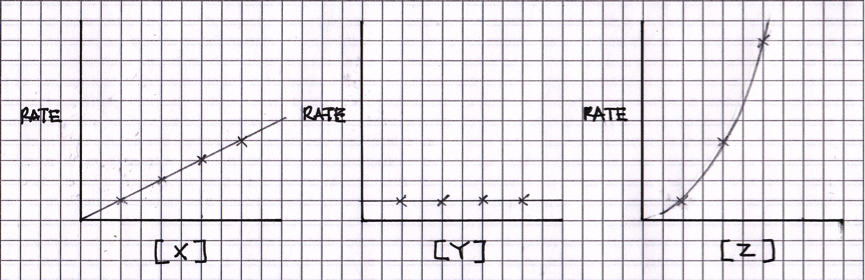
Which of the following shows the correct order of reaction with respect to [X], [Y] and [Z].?
For zero order reactants, changing concentration will have no effect on the rate, so [Y] is zero order. For first order reactants, changing concentration will change the rate proportionally (double concentration and double rate), so [X] is first order. For second order reactants, changing concentration will change the rate by the square of the concentration (double concentration and quadruple rate), so [Z] is second order.
[X] first order ; [Y] zero order ; [Z] second order is the correct answer.
Which of the following statements is true when following the concentration over time of a first order reactant in a chemical reaction?
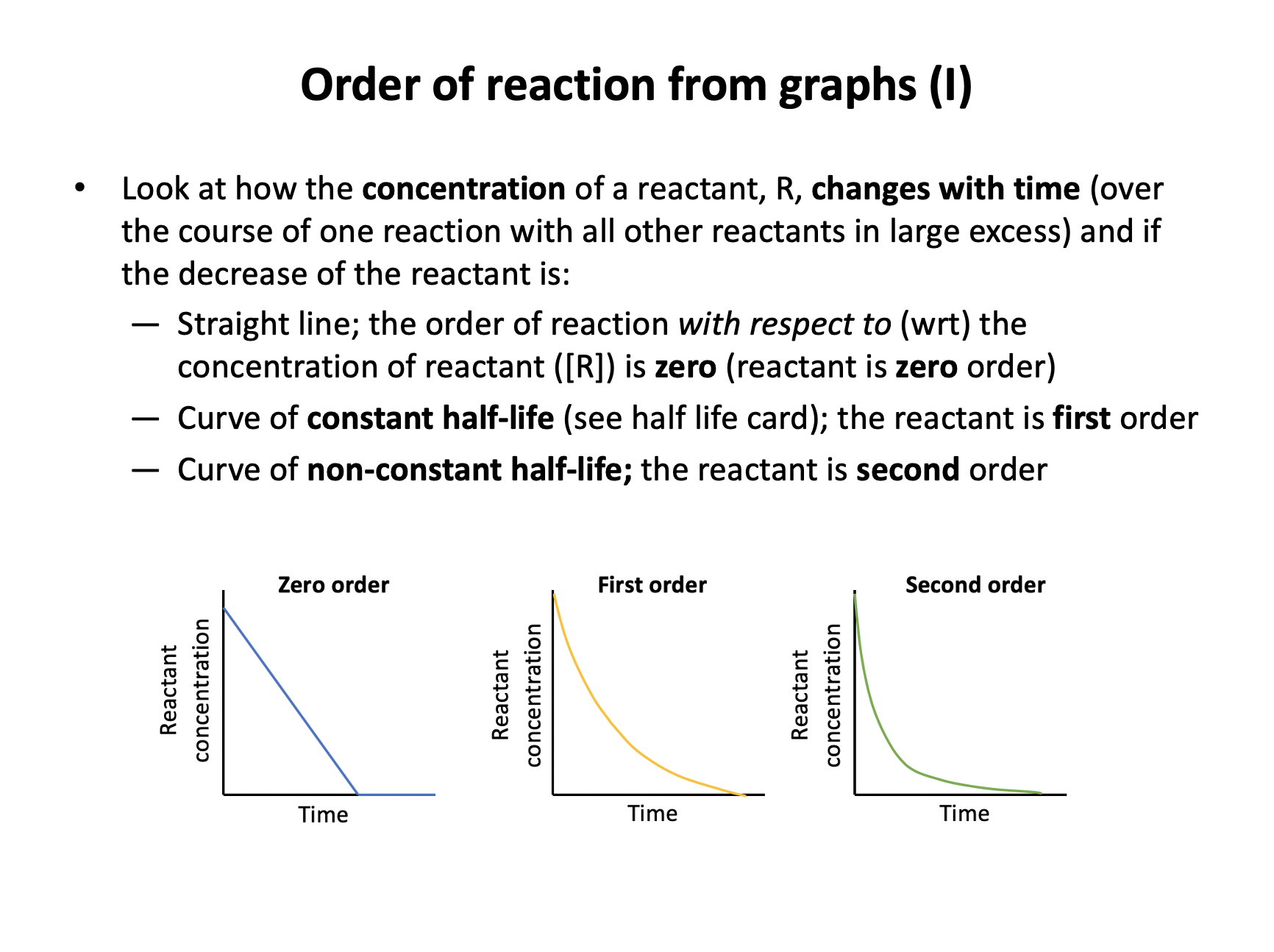
Half-life is the time taken for the concentration of a species to half in value. First order reactants always have constant half-lives, and a reactant will be used up as a reaction proceeds, so the correct answer is The concentration decreases with a constant half-life.
If the stoichiometric equation for a reaction is given thus:
2NO + H2 → N2 + H2O
What is the rate equation?
The rate equation can only be determined experimentally. It cannot be deduced from the stoichiometric equation. Thus the correct answer is 'unknown'. Although it is possible that any of the other answers given may be correct, we cannot know the answer without further information.
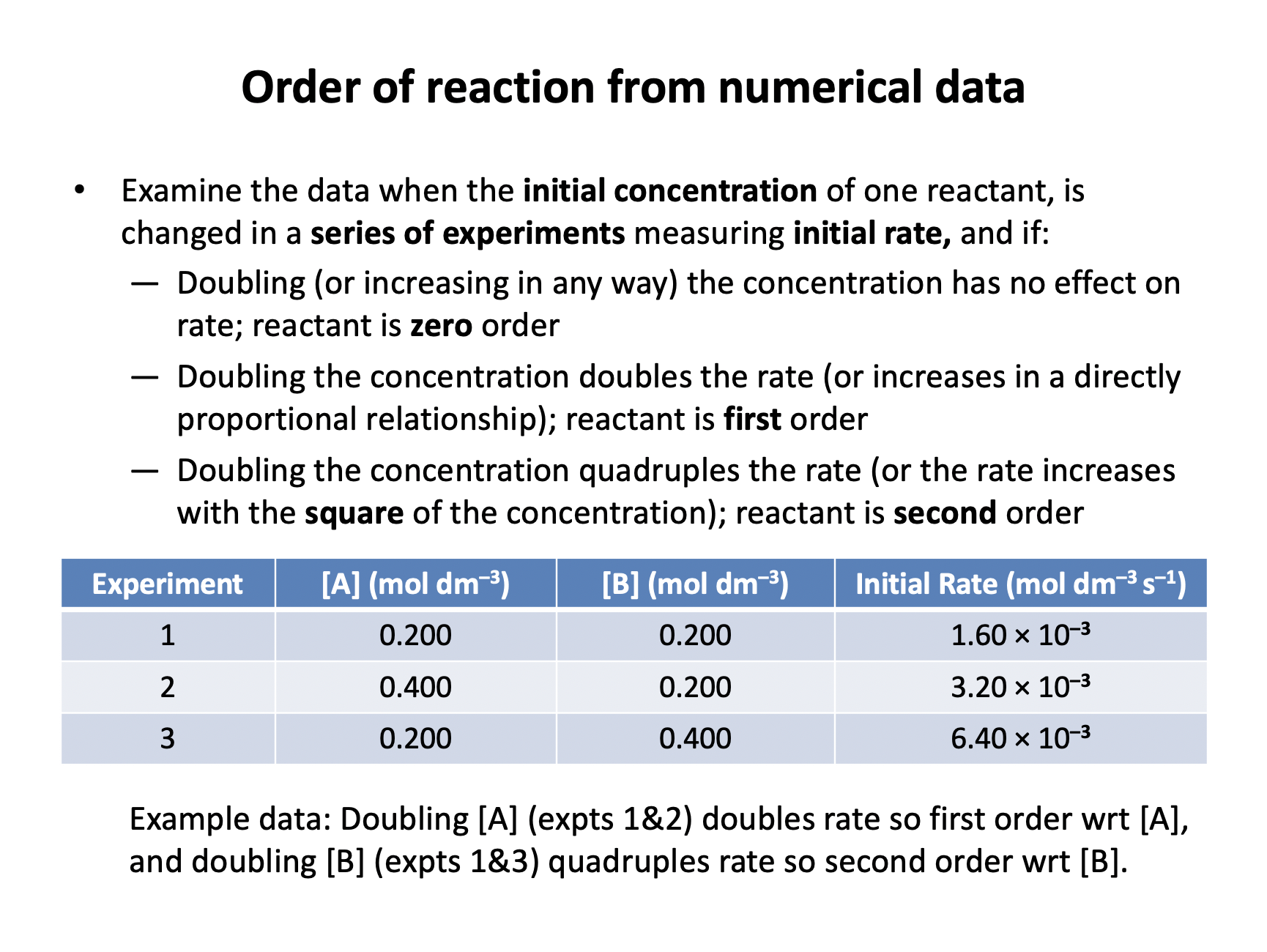
The following data was obtained for the chemical reaction: Y + Z → Products
| [Y] (mol dm–3) | [Z] (mol dm–3 ) | Initial Rate (mol dm–3 s–1) |
| 0.10 | 0.10 | 0.20 |
| 0.20 | 0.10 | 0.80 |
| 0.20 | 0.20 | 0.80 |
What is the order of reaction with respect to [Y] and the order of reaction with respect to [Z]?
The correct answer is that [Y] is 2nd order and [Z] is zero order. When the concentration of only Y is doubled (experiments 1 to 2) the rate quadruples. [Y] × 2 causes rate × 4, so [Y] is second order. When the concentration of only Z is doubled (experiments 2 to 3) the rate doesn't change, so [Z] is zero order.
The following data was obtained for the chemical reaction: X + Y → Products
| [X] (mol dm–3) | [Y] (mol dm–3 ) | Initial Rate (mol dm–3 s–1) |
| 0.20 | 0.20 | 4.0 × 10–3 |
| 0.20 | 0.60 | 3.6 × 10–2 |
| 0.30 | 0.20 | 6.0 × 10–3 |
What is the order of reaction with respect to X and the order of reaction with respect to Y?
The correct answer is that [X] is 1st order and [Y] is 2nd. When the concentration of Y is tripled (experiments 1 to 2) the rate increases nine fold (0.004 to 0.036 – make sure you understand standard form; to a positive power of ten moves the decimal place that many places to the right, negative power to the left). [Y] × 3 causes rate × 9, and 32=9, so [Y] is second order. When the concentration of X is multiplied by 1.5 (experiments 1 to 3) the rate also increases by 1.5 times (0.004 to 0.006). [X] × 1.5 causes rate × 1.5, and 1.51=1.5, so [X] is first order.
The following data was obtained for the chemical reaction: X + Y → Products
| [X] (mol dm–3) | [Y] (mol dm–3 ) | Initial Rate (mol dm–3 s–1) |
| 0.30 | 0.20 | 6.0 × 10–3 |
Given that the rate equation is:
Rate = k[X][Y]2
What is the numerical value of the rate constant?
k = Rate / [X][Y]2
k = 6.0 × 10–3 / 0.30 × (0.20)2
k = 0.006 / 0.3 × 0.04
k = 0.006 /0.012 = 0.5 is the correct answer
2: the fraction is the wrond way around
0.1: the square has been left out
0.33: 0.30 has been squared instead of 0.20
The following data was obtained for the chemical reaction: Y + Z → Products
| [Y] (mol dm–3) | [Z] (mol dm–3 ) | Initial Rate (mol dm–3 s–1) |
| 0.20 | 0.10 | 0.80 |
Given that the rate equation is:
Rate = k[Y]2
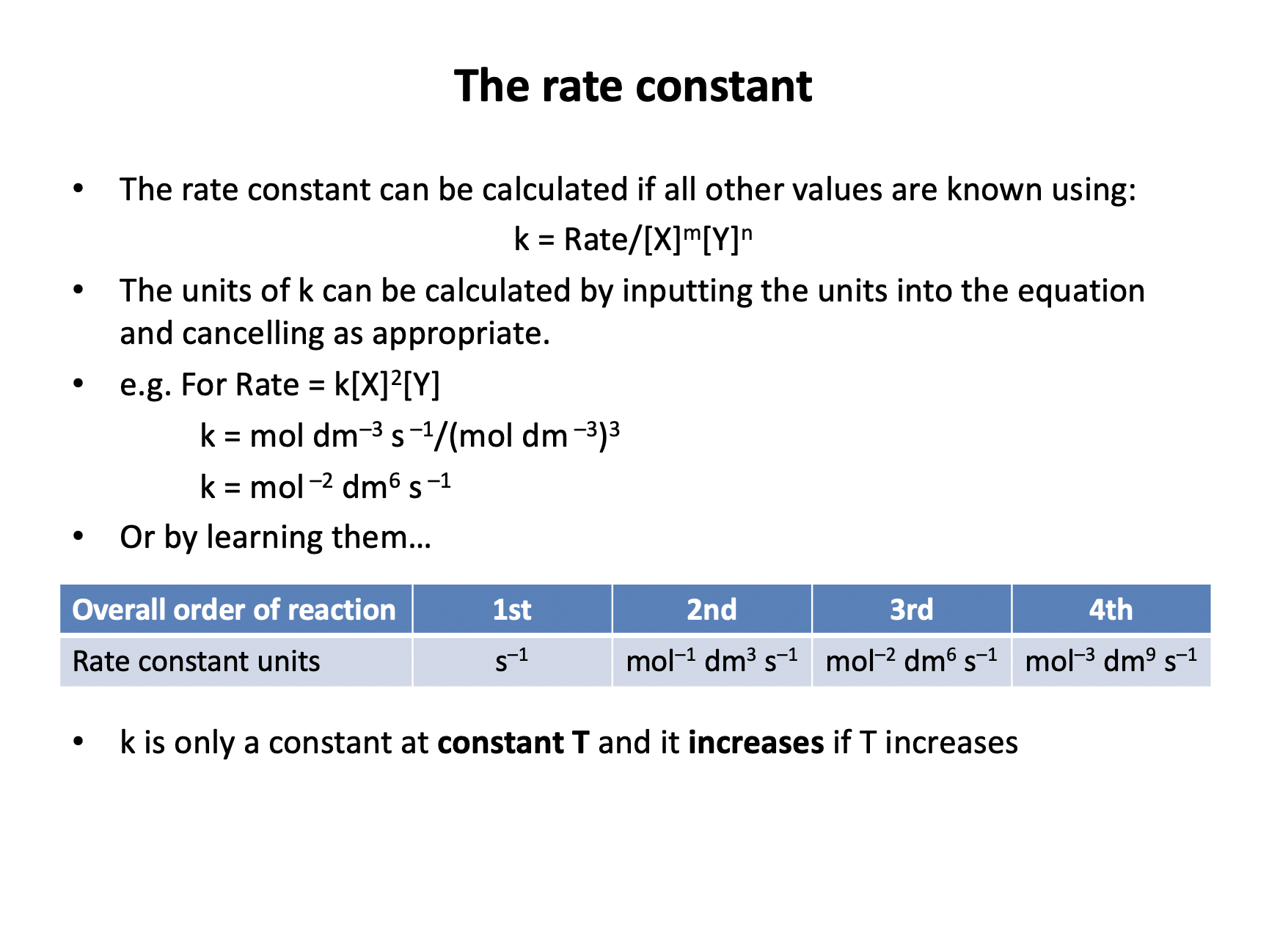
What are the units of the rate constant?
Units of the rate constant can be worked out by cancelling out in the equation in the same way as calculating the numerical value for the rate constant:
k = Rate / [Y]2
units = mol dm–3 s–1 / (mol dm–3)(mol dm–3)
So cancel one moles per decimetre cubed from top and bottom:
units = s–1 / mol dm–3
Move units from bottom to top and therefore invert the sign:
mol–1 dm3 s–1 is the correct answer.
Or they can be learnt:
Overall order of reaction | 1st | 2nd | 3rd | 4th |
Rate constant units | s–1 | mol–1 dm3 s–1 | mol–2 dm6 s–1 | mol–3 dm9 s–1 |
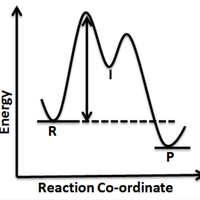
The mechanism below represents the SN1 reaction between 2-bromo-2-methyl propane and a hydroxide ion:
Step (i) (slow step): (CH3)3CBr → (CH3)3C+ + Br–
Step (ii) (fast step): (CH3)3C+ + OH– → (CH3)3COH
The hydroxide ion does not take part in step (i) and the bromide ion does not take part in step (ii).
What is the molecularity of the steps (i) and (ii) respectively?
Molecularity is the number of particles that react together in a given reaction/step. In step (i) the only reactant is (CH3)3CBr so the molecularity is 1 (a unimolecular step). In step (ii) the reactants are (CH3)3C+ and OH– so the molecularity is 2 (a bimolecular step). '1 and 2' is the correct answer.
Nitrogen dioxide and carbon monoxide react together according to the following stoichiometric equation:
NO2(g) + CO(g) → NO(g) + CO2(g)
Experimental data at high temperatures suggests the following mechanism for the reaction:
Step 1 (slow step): NO2(g) + NO2(g) → NO(g) + NO3(g)
Step 2 (fast step): NO3(g) + CO(g) → NO2(g) + CO2(g)
Given the mechanism above, what is the rate equation?
Only reactants that are in the rate determining step – slowest step – (or contribute to an intermediate that is in the rate determining step) can appear in the rate equation and therefore have an effect upon rate. NO2 appears twice in the slow/rate determining step and thus [NO2] has an order of 2. CO does not appear in slow/rate determining step and thus [CO] has an order of zero.
Nitrogen monoxide and oxygen react together according to the following stoichiometric equation:
2NO(g) + O2(g) → 2NO2(g)
Experimental data suggests the following mechanism for the reaction:
Step 1 (fast step): 2NO(g) ⇌ N2O2(g)
Step 2 (slow step): N2O2(g) + O2(g) → 2NO2(g)
Given the mechanism above, what is the rate equation?
Only reactants that are in the rate determining step – slowest step – or contribute to an intermediate that is in the rate determining step, can appear in the rate equation and therefore have an effect upon rate. O2 appears once in the slow/rate determining step and thus [O2] has an order of 1. N2O2 also appears in the slow/rate determining step; it is an intermediate. Intermediates cannot themselves appear in the rate equation (hence Rate = k[N2O2][O2] is incorrect) but the reactants that produce the intermediate do appear in the rate equation. And since two moles of NO produce the intermediate (N2O2), this is equivalent to appearing twice in the slow/rate determining step and thus [NO] has an order of 2. Rate = k[NO]2[O2] is the correct answer.
Paper 1
Core (SL&HL): Kinetics core (SL and HL) paper 1 questions
AHL (HL only): Kinetics AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Kinetics core (SL & HL) paper 2 questions
AHL (HL only): Kinetics AHL (HL only) paper 2 questions
How much of Rate expression and reaction mechanism have you understood?












 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn