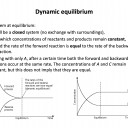
 Understanding how a chemical equilibrium functions is most important. If you can understand that the equilibrium will respond, whenever possible, to oppose any changes that are introduced, then you can work out the answer to any question by thinking it through!
Understanding how a chemical equilibrium functions is most important. If you can understand that the equilibrium will respond, whenever possible, to oppose any changes that are introduced, then you can work out the answer to any question by thinking it through!
Ensure you are confident using the terms below and learn the asterisked* definitions
dynamic equilibrium*, homogeneous equilibrium, heterogeneous equilibrium, reaction quotient, equilibrium position, equilibrium constant, backward reaction, forward reaction
Which of the following describes a system at equilibrium?
1: The system has no exchange with the surroundings.
2: The rate of the forward and backward reactions are equal.
3: The concentration of the reactants are the same as the concentration of the products.
'1 and 2 only' is the correct answer, since an equilibrium is defined as being closed (1) and having equal rate of reaction in both directions (2), but the concentrations of reactants and products do not need to be the same (3), and rarely are. The third statement might better read 'the concentrations of reactants and products remain constant', which they do, but not the same as each other.
Which is the correct equilibrium expression for the equilibrium as given below?
2SO2 + O2 ⇌ 2SO3
The equilibrium constant is defined as being the product of the concentrations of the products (each to the power of the number of moles) over the product of the concentrations of the reactants (each to the power of the number of moles), e.g. for a reaction:
aA + bB ⇌ cC + dD
![]()
Given the equilibrium for the Haber process N2(g) +3H2(g) ⇌ 2NH3(g) and the equilibrium expresssion:
\(K_c = {{[NH_3]^2} \over [N_2][H_2]^3}\)
If Kc = 0.044 at 200°C what can be said about the position of equilibrium at 200°C?
The value of Kc is 0.044. This number is less than 1, and indicates that the bottom of the fraction is larger than the top. This means that there are more reactants than products in the equilibrium mixture, and this is what 'lies to the left' means.
Thus the correct answer is 'The equilibrium lies to the left; there are more reactants than products in the equilibrium mixture'.
Given the equilibrium for the Haber process N2(g) +3H2(g) ⇌ 2NH3(g) and the equilibrium expresssion:
\(K_c = {{[NH_3]^2} \over [N_2][H_2]^3}\)
If Kc = 0.044 at 200°C, what will happen to the position of equilibrium and to the value of Kc if a catalyst (finely divided iron) is added?
Remember that Kc is constant at constant temperature. So addition of a catalyst will not affect Kc.
Addition of a catalyst will not affect the position of an equilbrium either. It will decrease the time it takes for a reaction to reach equilibrium, because it will speed up the rate of the foward and backward reactions equally, but will not alter the position of the equilibrium when it gets there.
Given the equilibrium for the Haber process N2(g) +3H2(g) ⇌ 2NH3(g) and the equilibrium expresssion:
\(K_c = {{[NH_3]^2} \over [N_2][H_2]^3}\)
If Kc = 0.044 at 200°C and ΔH = −92 kJ, what will happen to the value of Kc if the temperature is increased?
Increasing temperature will favour the endothermic direction, which is the reverse direction in this case (a negative ΔH indicates an exothermic forward reaction). The equilibrium will shift to the left to give a greater proportion of reactants. A greater proportion of reactants will make the bottom of the fraction greater and will therefore reduce the value of Kc.
The value of Kc will be < 0.044.
Which is the best description of Q, the reaction quotient?
The correct answer is 'Q is the same as the Kc expression, but not necessarilly with equilibrium concentration values'.
Q is useful when a reaction is thrown out of equilibrium e.g.
A + B ⇌ C
\(K_c = {{[C]} \over [A][B]}\) at equilibrium, and
\(Q = {{[C]} \over [A][B]}\)
The reaction is at equilibrium (Kc = Q), and then more A is added: The reaction is suddenly thrown out of equilibrium and now Q will be smaller than Kc (since [A] has increased and it is on the bottom of the fraction). The equilibrium will now shift to the right, decreasing the amount of A (and B) and increasing the amount of C, until Q once more equals the value of Kc. Equilibrium is re-attained.
Consider the reaction given below at equilibrium:
N2O4(g) ⇌ 2NO2(g)
If more N2O4 gas is pumped into the container, what will happen to the value of Q, the reaction quotient?
The correct answer is 'Q will initially decrease; the equilibrium will then shift causing Q to increase again until Q = Kc'.
\(K_c = {{[NO_2]^2} \over [N_2O_4]}\) ; this is also the expression for Q.
The reaction is at equilibrium (Kc = Q), and then more N2O4 is pumped in: The reaction is suddenly thrown out of equilibrium and now Q will be smaller than Kc (since [N2O4] has increased and it is on the bottom of the fraction). The equilibrium will now shift to the right, decreasing the amount of N2O4 and increasing the amount of NO2, until Q once more equals the value of Kc. Equilibrium is re-attained.
Given the equilibrium for the Haber process N2(g) +3H2(g) ⇌ 2NH3(g) and the equilibrium expresssion:
\(K_c = {{[NH_3]^2} \over [N_2][H_2]^3}\)
If Kc = 0.044 at 200°C what will happen to the position of equilibrium and to the value of Kc if the pressure is increased (by reducing the total volume)?
Remember that Kc is constant at constant temperature.
The value of Kc will therefore remain as 0.044 when the volume decrease causes an increase in pressure, but the increase in pressure will favour the side of the equilibrium with fewest moles of gas (2 moles of the right) so the equilibrium will shift right.
Decreasing the volume (and thus increasing total pressure) causes the concentration values to each increase. There are more concentration terms on the bottom than the top of the fraction, so the bottom becomes bigger; decreasing the volume throws the reaction out of equilibrium and Q becomes smaller than Kc. Thus the equilibrium shifts to increase the top of the fraction (more ammonia, NH3); that is a shift to the right, until Q once more equals Kc (0.044).
\(Q = {{[NH_3]^2} \over [N_2][H_2]^3}\)
Paper 1
Core (SL&HL): Equilibrium core (SL and HL) paper 1 questions
AHL (HL only): Equilibrium AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Equilibrium core (SL & HL) paper 2 questions
AHL (HL only): Equilibrium AHL (HL only) paper 2 questions
How much of Equilibrium have you understood?









 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn