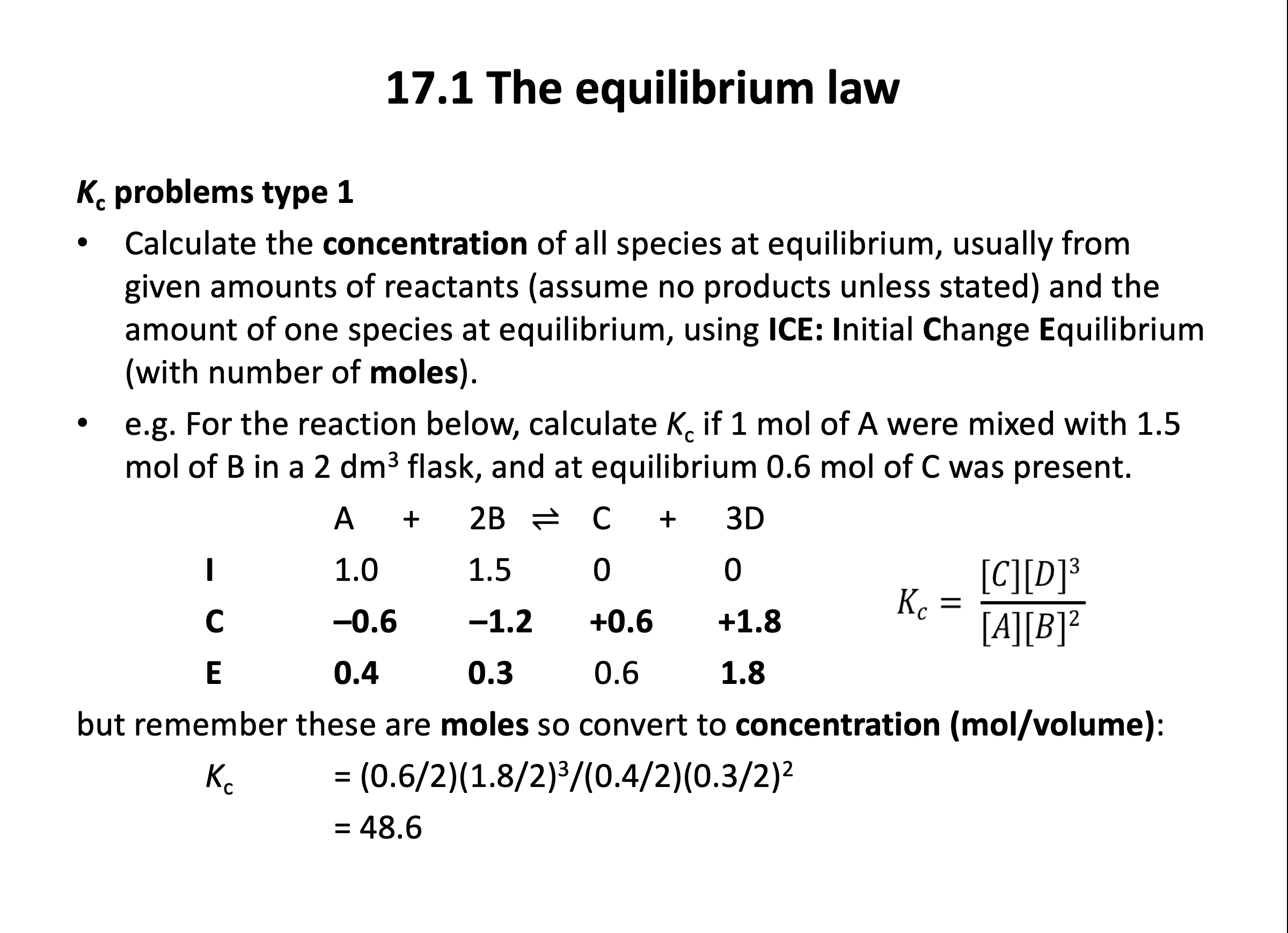
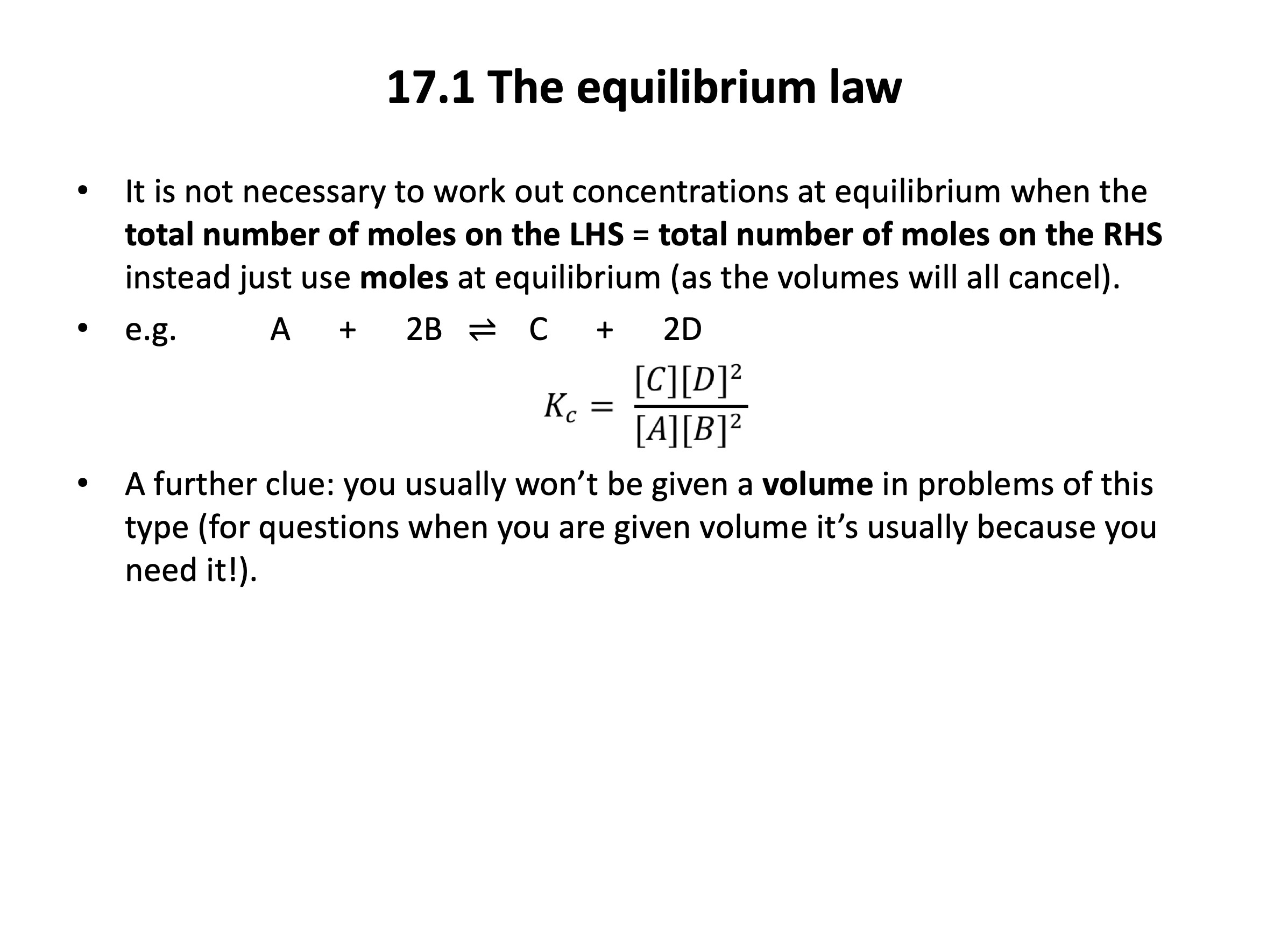
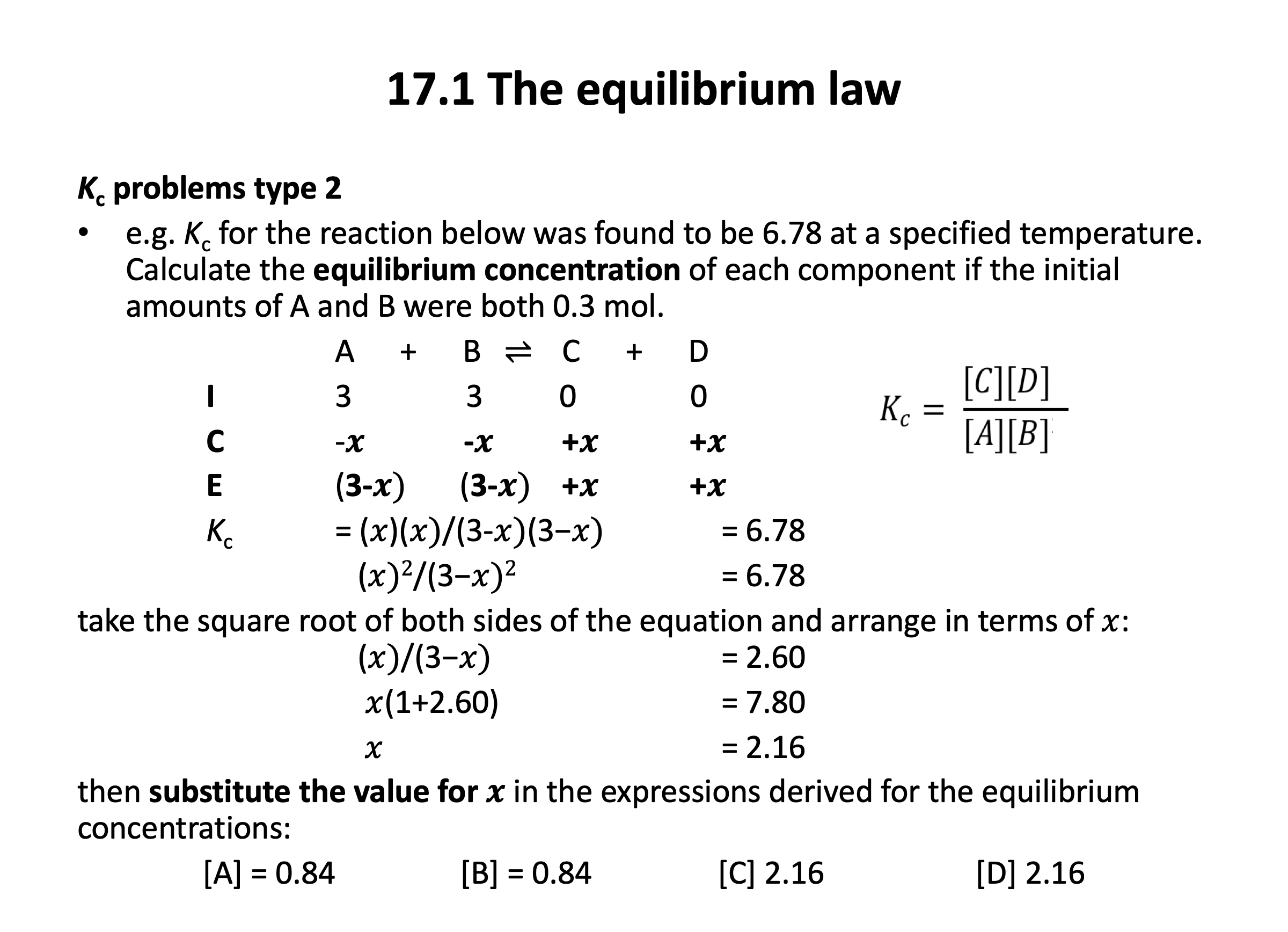
 The application of numbers to equilibrium can be challenging, but if you are confident with mole calculations (Stoichiometric relationships), and you also have a sound understanding of how an equilibrium functions (Equilibrium) then it will feel a lot easier. It is worth getting plenty of practice applying the ICE (initial, change, equilibrium) method with the questions in the quiz below and in the Qbank.
The application of numbers to equilibrium can be challenging, but if you are confident with mole calculations (Stoichiometric relationships), and you also have a sound understanding of how an equilibrium functions (Equilibrium) then it will feel a lot easier. It is worth getting plenty of practice applying the ICE (initial, change, equilibrium) method with the questions in the quiz below and in the Qbank.
A gaseous sample of pure iodine monobromide (IBr) is heated and allowed to reach equilibrium at 125°C.
2IBr(g) ⇌ I2(g) + Br2(g)
Initially there was only 0.50 mol of iodine monobromide present.
At equilibrium 0.30 mol of iodine monobromide could be detected.
How many moles of bromine (Br2) are in the equilibrium mixture?
Using the initial-change-equilibrum (ICE) moles table:
| 2IBr(g) | ⇌ | I2(g) | + | Br2(g) | |
| I | 0.50 | 0 | 0 | ||
| C | 1st −0.20 | 2nd +0.10 | 2nd +0.10 | ||
| E | 0.30 | 3rd 0.10 | 3rd 0.10 | ||
The non-bold information is given in the question.
We have initial and equilibrium moles of IBr so the 1st step is to calculate the change in IBR: −0.20
If we have the change in IBr we can then calculate the change in I2 and Br2 (2nd): The mole ratio is 2:1, so two moles of IBr used up will produced one mole of iodine and one mole of bromine. Thus 0.20 mol of IBr used up will produced 0.10 mol of both iodine and bromine.
We now have the initial moles and change for Br2, so lastly (3rd) we can calculate the moles at equilibrium: 0+0.10=0.10 mol
The correct answer is 0.10
A gaseous sample of pure dinitrogen tetroxide (N2O4) is heated and allowed to reach equilibrium at 50°C.
N2O4(g) ⇌ 2NO2(g)
If 0.500 mol of dinitrogen tetroxide (N2O4) was initially present and at equilibrium 0.050 mol of nitrogen dioxide (NO2) could be detected.
How many moles of dinitrogen tetroxide are present in the equilibrium mixture?
Using the initial-change-equilibrum (ICE) moles table:
| N2O4(g) | ⇌ | 2NO2(g) | |
| I | 0.500 | 0 | |
| C | 2nd −0.025 | 1st +0.050 | |
| E | 3rd 0.475 | 0.050 | |
The non-bold information is given in the question.
We have initial and equilibrium moles of NO2 so the 1st step is to calculate the change in NO2: +0.050.
If we have the change in NO2 we can then calculate the change in N2O4 (2nd): The mole ratio is 1:2, so two moles of NO2 produced will use up one mole of N2O4. Thus 0.050 moles of NO2 produced uses up (half the amount) 0.025 mol of N2O4.
We have the initial moles and change for N2O4, so lastly (3rd) we can calculate the moles of N2O4 at equilibrium: 0.500−0.025=0.475 mol
The correct answer is 0.475
A gaseous sample of pure dinitrogen tetroxide (N2O4) is heated in a 600cm3 flask and allowed to reach equilibrium at 60°C.
N2O4(g) ⇌ 2NO2(g)
If 0.400 mol of dinitrogen tetroxide (N2O4) was initially present and at equilibrium 0.200 mol of nitrogen dioxide (NO2) could be detected.
What is the value of Kc under these conditions?
Using the initial-change-equilibrum (ICE) moles table:
| N2O4(g) | ⇌ | 2NO2(g) | |
| I | 0.400 | 0 | |
| C | 2nd −0.100 | 1st +0.200 | |
| E | 3rd 0.300 | 0.200 | |
The moles of each species at equilibrium must be calculated. The non-bold information is given in the question.
We have initial and equilibrium moles of NO2 so the 1st step is to calculate the change in NO2: +0.200.
If we have the change in NO2 we can then calculate the change in N2O4 (2nd): The mole ratio is 1:2, so two moles of NO2 produced will use up one mole of N2O4. Thus 0.200 mol of NO2 produced uses up 0.100 mol of N2O4.
We have the initial moles and change for N2O4, so lastly (3rd) we can calculate the moles of N2O4 at equilibrium: 0.400−0.100=0.300 mol
These mol values then need to be converted to concentrations (using the volume of the flask given in the question: 600cm3 which is 0.600dm3).
Conc N2O4 = 0.300/0.600 = 0.500moldm−3
Conc NO2 = 0.300/0/600 = 0.333moldm−3
These values then need to be put into the equilibrium expression:
\(K_c = {{[NO_2]^2} \over [N_2O_4]}\)
Kc = (0.333)2/0.500 = 0.111/0.500 = 0.222
The correct answer is therefore 0.222. Units are not required for Kc since it is a ratio.
Incorrect answers
0.666 is obtained if the [NO2] value is not squared.
0.133 is obtained if the mol values are not converted to concentrations using the total volume of 0.600dm3.
0.200 is obtained if the mole ratio is used as 1:1 in the equation and the mol values are not converted to concentrations using the total volume of 0.600dm3.
A gaseous sample of pure iodine monobromide (IBr) is heated and allowed to reach equilibrium at 125°C.
2IBr(g) ⇌ I2(g) + Br2(g)
Initially there was 0.500 mol of iodine monobromide present.
At equilibrium 0.400 mol of iodine monobromide could be detected.
What is the value of Kc (to three sig figs) under these conditions?
Using the initial-change-equilibrum (ICE) moles table:
| 2IBr(g) | ⇌ | I2(g) | + | Br2(g) | |
| I | 0.500 | 0 | 0 | ||
| C | 1st −0.100 | 2nd +0.050 | 2nd +0.050 | ||
| E | 0.400 | 3rd 0.050 | 3rd 0.050 | ||
The moles of each species at equilibrium must be calculated. The non-bold information is given in the question.
We have initial and equilibrium moles of IBr so the 1st step is to calculate the change in IBR: −0.100
If we have the change in IBr we can then calculate the change in I2 and Br2 (2nd): The mole ratio is 2:1, so two moles of IBr used up will produced one mole of iodine and one mole of bromine. Thus 0.100 mol of IBr used up will produced 0.050 mol of both iodine and bromine.
We now have the initial moles and change for I2 and Br2, so lastly (3rd) we can calculate the moles at equilibrium: 0.050
Often these mol values then need to be converted to concentrations (using the volume of the flask given in the question), but here there is no volume given in the question because there are two concentration terms on top of the Kc expression and two on the bottom; the volumes cancel.
\(K_c = {{[I_2][Br_2]} \over [IBr]^2}\)
The equilibrium mol vaules can therefore be inserted directly into the expression as 'concentrations':
Kc = 0.050×0.050/(0.400)2= 0.025/0.160 = 0.015625
The correct answer is therefore 0.016 to 3 sig figs. Units are not required for Kc since it is a ratio.
Incorrect answers
0.006 is obtained if the [IBr] value is not squared.
0.063 is obtained if the mole ratio is used as 1:1 in the equation and 0.100 mol of iodine and bromine are assumed to be present at equilibrium.
0.025 is obtained if the [IBr] value is not squared and the mole ratio is used as 1:1 in the equation.
An esterification reaction is allowed to reach equilibrium at room temperature:
carboxylic acid(aq) + alcohol(aq) ⇌ ester(aq) + water(l)
The stoichiometric equation is a 1:1 ⇌ 1:1 ratio.
Initially there was 0.400 mol of carboxylic acid and 0.400 mol of alcohol present.
If at equilibrium the value of Kc was found to be 4.00 what is the concentration of the alcohol at equilibrium (in moldm−3) to 3 sig figs?
Note immediately that there is no volume given in the question because there are two concentration terms on top of the Kc expression and two on the bottom; the volumes cancel.
\(K_c = {{[ester][water]} \over [acid][alcohol]}\)
Thus the mol values can be used directly in the Kc expression.
Using the initial-change-equilibrum (ICE) moles table:
| carboxylic acid(aq) | + | alcohol(aq) | ⇌ | ester(aq) | + | water(l) | |
| I | 0.400 | 0.400 | 0 | 0 | |||
| C | 0.400−x | 0.400−x | +x | +x | |||
| E | 0.400−x | 0.400−x | x | x | |||
The non-bold information is given in the question.
Algebra is needed. The mole ratio is 1:1 ⇌ 1:1 so the decrease in moles of each reactant will be the same as the increase in moles of each product.
Inserting the equilibrium mol values into the expression, and using the Kc value given in the question:
\(4.00 = {{x^2} \over (0.400-x)^2}\)
Taking square roots of each side of the equation:
\(2.00 = {{x} \over (0.400-x)}\)
And rearranging in terms of x:
x = 2.00(0.400−x)
x = 0.800−2x
3x = 0.800
x = 0.266
The moles of alcohol is 0.400−x = 0.400−0.266 = 0.133 (and the volumes cancel so this is the value for concentration too)
Thus the correct answer is 0.133
Incorrect answers
0.266 is the value of x, but not the value for the alcohol.
0.320 is the value of x obtained when the 4.00 value for Kc is not square rooted, but the fraction is square rooted.
0.080 is the value for the alcohol when x is 0.320 (that is obtained when the 4.00 value for Kc is not square rooted, but the fraction is square rooted).
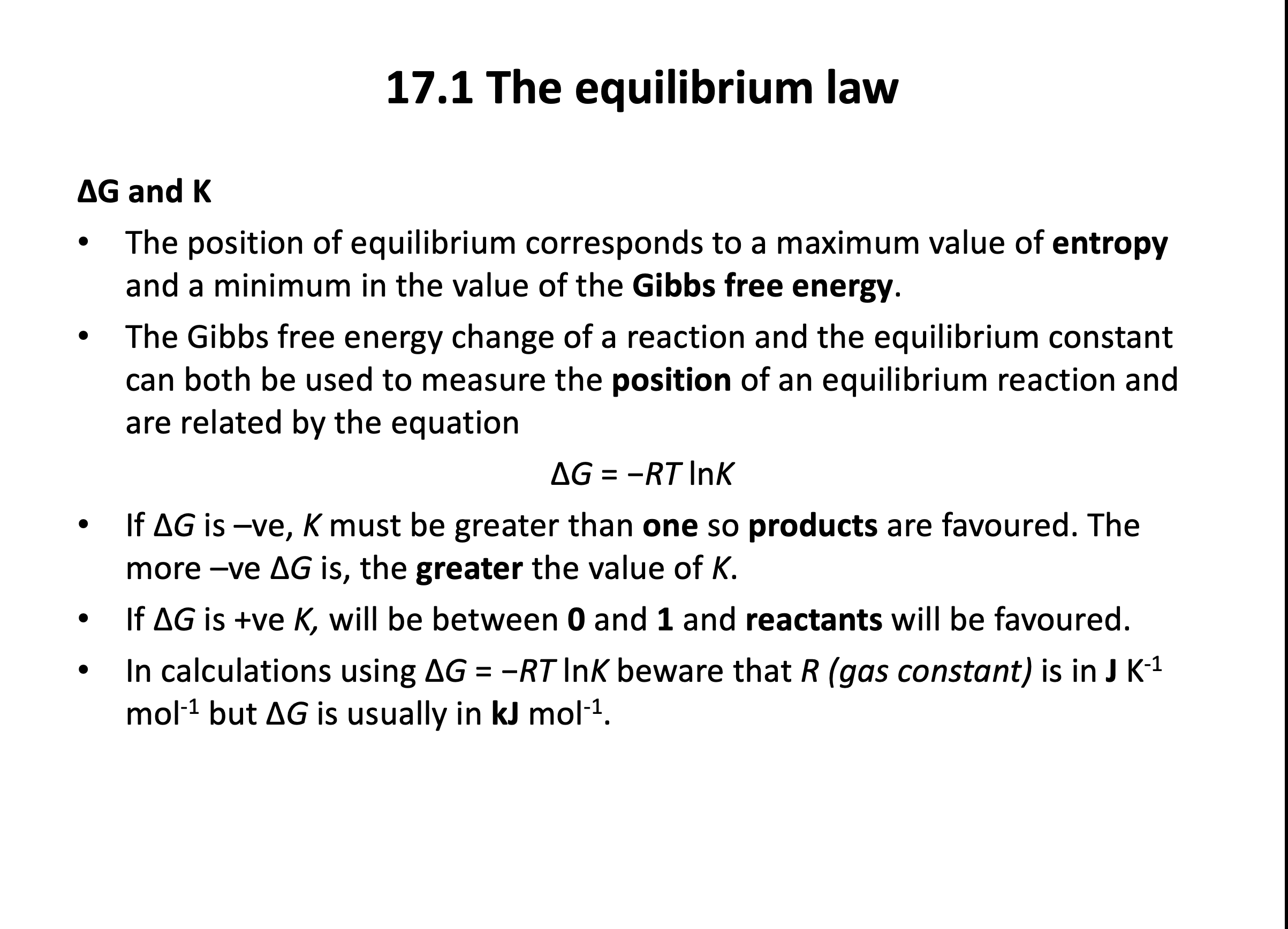
The position of equilibrium for a reaction corresponds to:
You need to learn that:
The position of equilibrium corresponds to a maximum value of entropy and a minimum value of Gibb's free energy.
The standard Gibb's free energy for the equilibrium reaction given below is −12.9kJmol−1 at 127°C.
N2O4(g) ⇌ 2NO2(g)
What is the value of the equilibrium constant?
ΔG° = −RTlnK is given in the data book.
R is the gas constant 8.31 JK−1mol−1 (also given in the data book).
T is temperature in K. 127°C is 400K (+273)
The critical thing to remember here is that ΔG is in kJ and R is in J, so best to multiply the ΔG value by 1000 to get everything into Joules.
ΔG° = −RTlnK becomes
−12900 = −8.31×400×lnK
Rearranging:
lnK = 12900 / 8.31×400 = 3.88...
K = e3.88... = 48.5
K does not require units (it is a ratio).
The correct answer is 48.5
Incorrect answers
20.3×104 is obtained by using 127 for T instead of 400.
1.00 is obtained by not correcting for units by multiplying by 1000: using −12.9, instead of −12900 for ΔG.
3.88 is the value of lnK (not K).
Paper 1
Core (SL&HL): Equilibrium core (SL and HL) paper 1 questions
AHL (HL only): Equilibrium AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Equilibrium core (SL & HL) paper 2 questions
AHL (HL only): Equilibrium AHL (HL only) paper 2 questions
How much of The equilibrium law have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn