 This section follows on directly from Theories and properties of acids and bases 8.1 and 8.2, so it is worth ensuring a thorough grasp of that content before beginning revision here. The concepts are relatively straightforward, but the introduction of pH (a logarithmic scale) can be a challenge mathematically. Ensure that you know your calculator well!
This section follows on directly from Theories and properties of acids and bases 8.1 and 8.2, so it is worth ensuring a thorough grasp of that content before beginning revision here. The concepts are relatively straightforward, but the introduction of pH (a logarithmic scale) can be a challenge mathematically. Ensure that you know your calculator well!
Ensure you are confident using the terms below and learn the asterisked* definitions
A strong acid or alkali*, A weak acid or alkali*, pH*, The ionic product of water Kw*, An acid-base indicator, Acid deposition
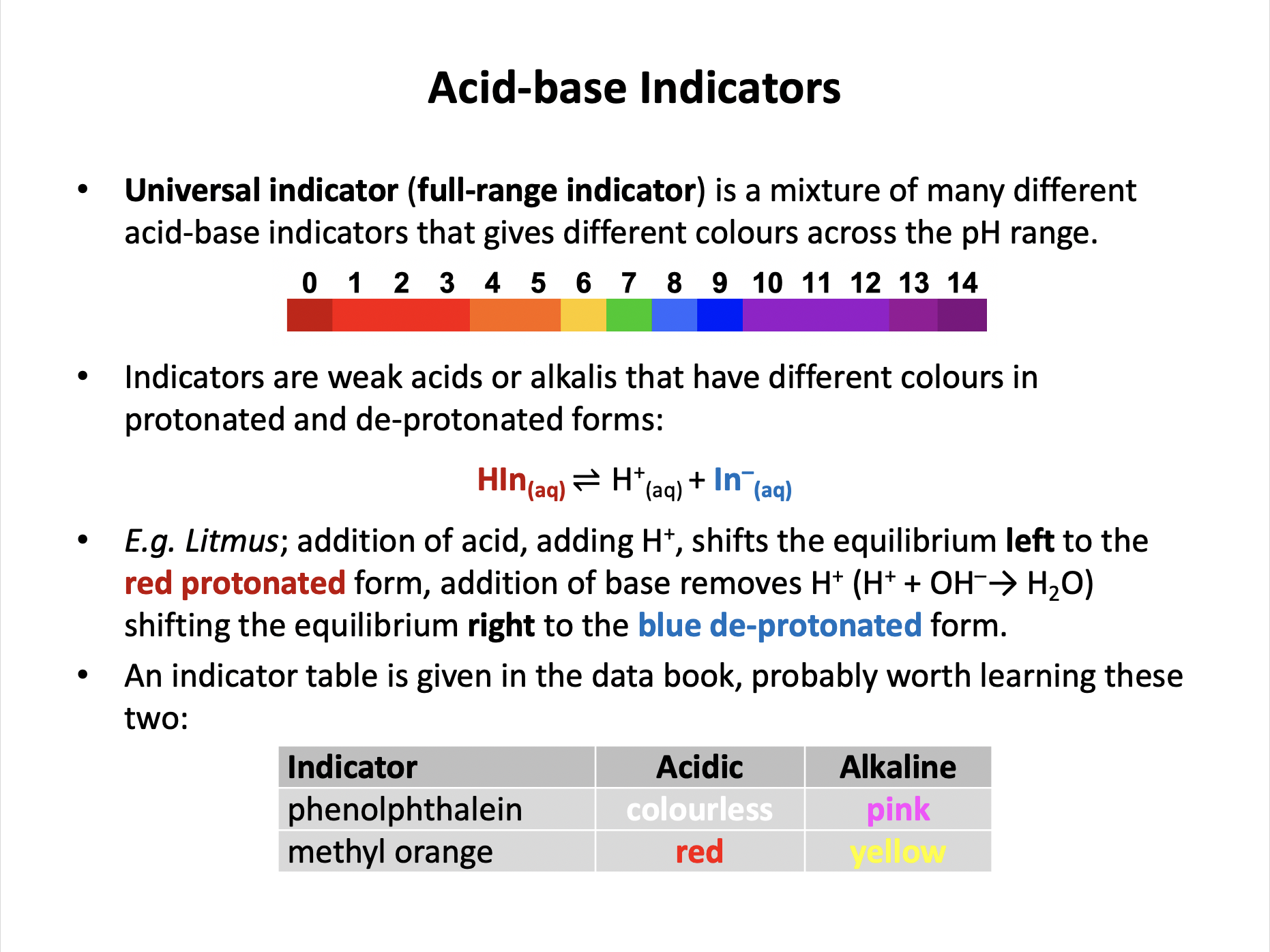
A sample with a pH of 3.0 was tested with methyl orange. A seperate sample of the same pH was tested with phenolphthalein. What colour would each indicator turn?
The sample is acidic.
Acids cause methyl orange to turn red (colour change 3.1-4.4), and cause phenolphthalein to turn colourless (colour change 8.3-10.0).
There is a table of indicator colours in the data book.
Calculator question: The concentration of hydroxide ions, [OH−], in a sample of river water was found to be 1.5 × 10−8 mol dm−3. What is the concentration of hydrogen ions, [H+], in this sample (2 sf)?
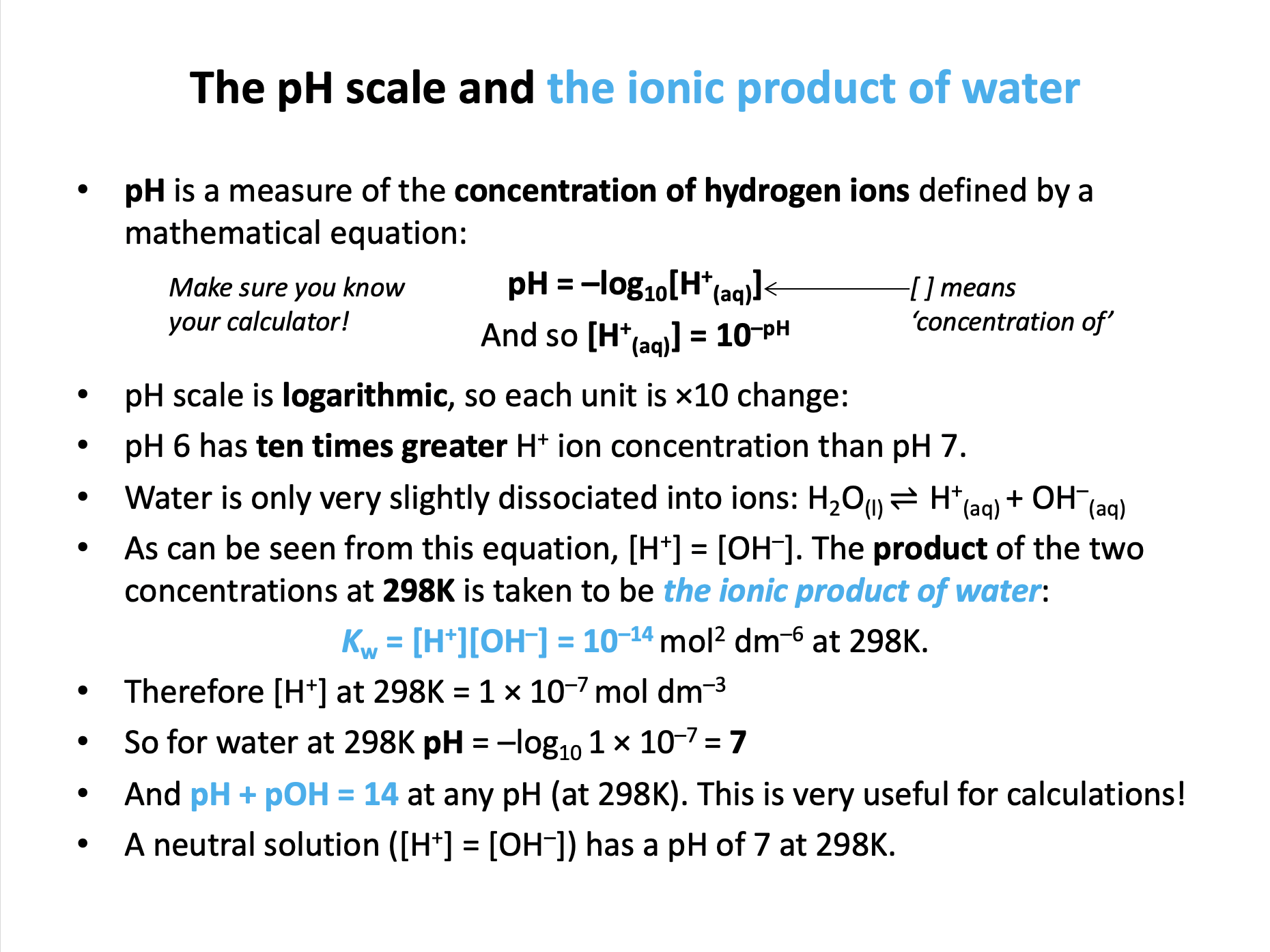
The relationship between [OH−] and [H+] is given by the ionic product of water (which should be learned):
Kw = [OH−] × [H+] = 1 × 10−14 mol2 dm−6
Therefore [H+] = Kw / [OH−] = 1 × 10−14 / 1.5 × 10−8 = 6.7 × 10−7
6.7 × 10−7 is therefore the correct answer.
Incorrect answers
1.5 × 106 is the answer if the fraction is inverted ([OH−] / Kw)
−1.5 × 10−8 is the answer if an incorrect addition expression is used for Kw.
0.67 is the answer if the powers of 10 are omitted.
Calculator question: The concentration of hydrogen ions, [H+], in a sample of hydrochloric acid is found to be 1.4 × 10−3. What is the pH of this sample (2sf)?
pH can be found by pH = −log10[H+]
pH = −log10 1.7 × 10−3 = 2.854 = 2.9 (2sf)
2.9 is therefore the correct answer.
Incorrect answers
2.8 is a rounding error (fives round up by convention)
−2.9 is missing out the minus sign in the equation
−2.8 is missing out the minus sign in the equation and a rounding error (fives round up by convention)
Calculator question: The concentration of hydroxide ions, [OH−], in a sample of sodium hydroxide was found to be 2.50 × 10−2. What is the pH of this sample (3sf)?
The relationship between [OH−] and [H+] is given by the ionic product of water (which should be learned):
Kw = [OH−] × [H+] = 1 × 10−14 mol2 dm−6
Therefore [H+] = Kw / [OH−] = 1 × 10−14 / 2.5 × 10−2 = 4.00 × 10−13
pH can be found by pH = −log10[H+]
pH = −log10 4.00 × 10−13 = 12.397 = 12.4 (3sf)
12.4 is therefore the correct answer.
(This can also be solved by using pH + pOH = 14, finding pOH first (1.60) and taking away from 14 to give 12.4)
Incorrect answers
1.60 is pOH −log10[OH−]
The negative numbers are errors in missing out the minus sign for the pH expression (or an inversion of the Kw expression).Calculator question: The concentration of a sample of hydrochloric acid is 0.45 mol dm−3.
What is the pH of this sample (2sf)?
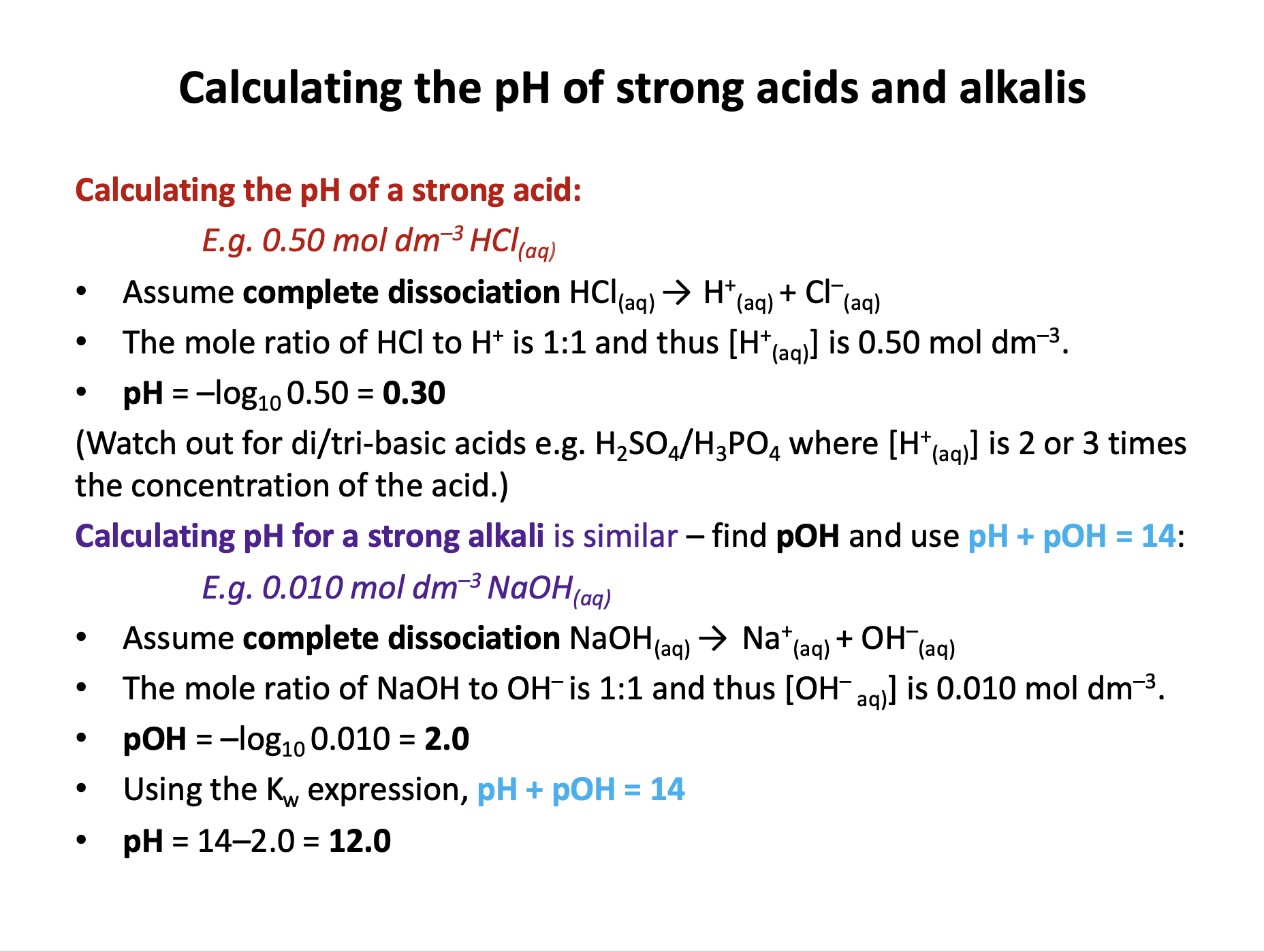
Strong acids completely dissociate: HCl(aq) → H+(aq) + Cl−(aq)
One mole of HCl gives one mole of H+ ions, so [HCl] = [H+]
pH can be found by pH = −log10[H+]
pH = −log10 0.45 = 0.346 = 0.35 (2sf)
0.35 is therefore the correct answer.
Incorrect answers
0.34 is a rounding error
−0.35 is missing out the minus sign in the equation
−0.34 is missing out the minus sign in the equation and a rounding error
Calculator question: The concentration of a sample of sodium hydroxide is 0.0800 mol dm−3.
What is the pH of this sample (3sf)?
Strong alkalis completely dissociate: NaOH(aq) → Na+(aq) + OH−(aq)
One mole of NaOH gives one mole of OH− ions, so [NaOH] = [OH−]
The relationship between [OH−] and [H+] is given by the ionic product of water (which should be learned):
Kw = [OH−] × [H+] = 1 × 10−14 mol2 dm−6
Therefore [H+] = Kw / [OH−] = 1 × 10−14 / 0.0800 = 1.25 × 10−13
pH can be found by pH = −log10[H+]
pH = −log10 1.25 × 10−13 = 12.903... = 12.9 (3sf)
12.9 is therefore the correct answer.
(This can also be solved by using pH + pOH = 14, finding pOH first (1.10) and taking away from 14 to give 12.9)
Incorrect answers
1.10 is pOH −log10[OH−]
The negative numbers are errors in missing out the minus sign for the pH expression (or an inversion of the Kw expression).Calculator question: The concentration of a sample of sulfuric acid is 0.030 mol dm−3.
What is the pH of this sample (2sf)?
Strong acids completely dissociate: H2SO4(aq) → 2H+(aq) + SO4−(aq)
One mole of H2SO4 gives two moles of H+ ions, so 2×[H2SO4] = [H+] = 0.060
pH can be found by pH = −log10[H+]
pH = −log10 0.060 = 1.2218... = 1.2 (2sf)
1.2 is therefore the correct answer.
Incorrect answers
1.5 is taking [H+] = 0.060
1.8 is taking [H+] = 0.015 (dividing conc. of acid by two instead of multiplying by two)
0.030 is the concentration of the acid
Which of the following are true statements about a 1 mol dm−3 aqueous solution of hydrochloric acid?
1: It fully dissociates into ions in aqueous solution
2: It will have a pH greater than 7
3: It is a good conductor of electricity
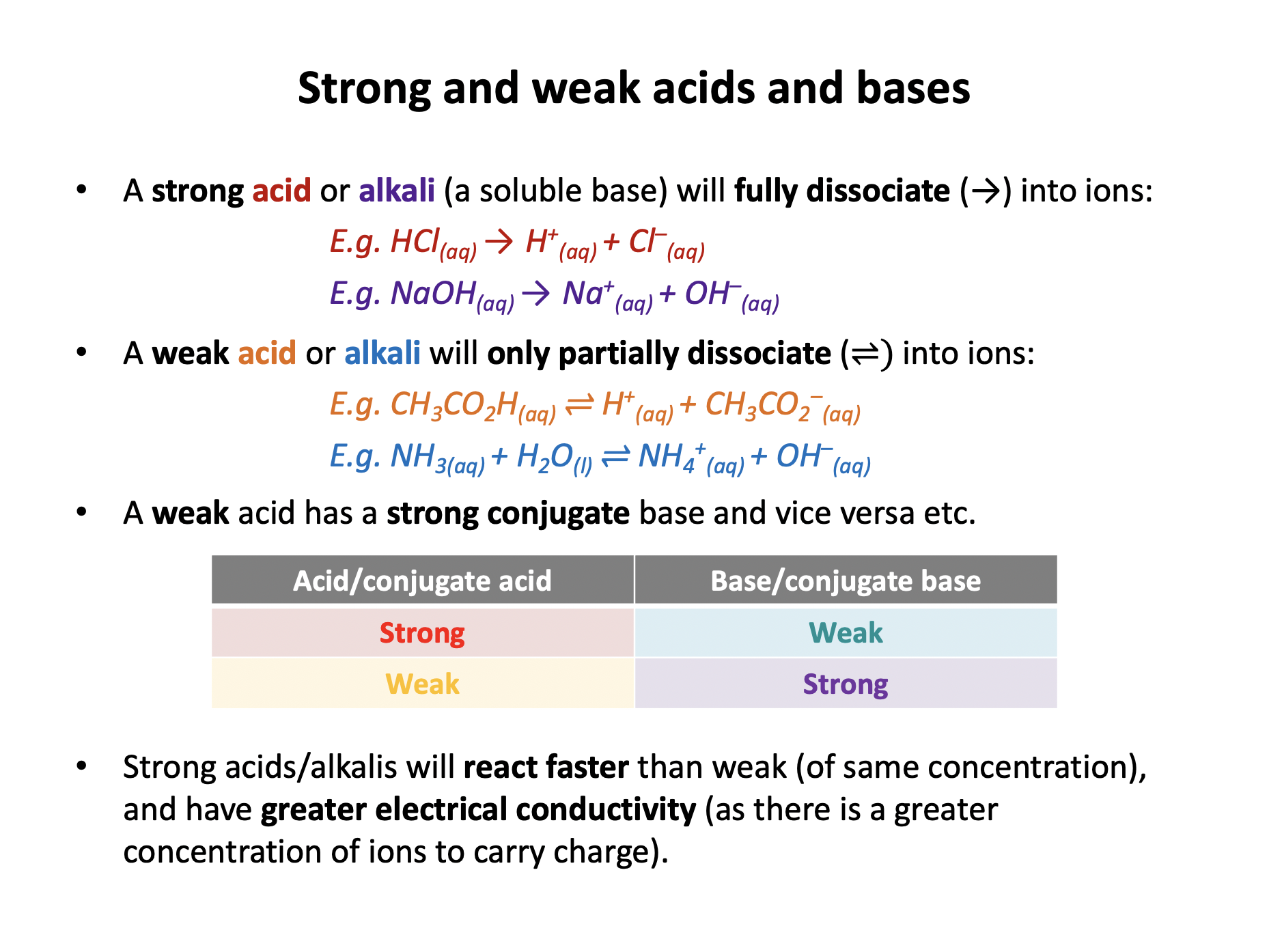
Strong acids and alkalis fully dissociate into ions in aqueous solution, weak acids and alkalis only partially dissociate. Strong acids and alkalis will react faster than weak acids and alkalis, and will have greater electrical conductivity.
Hydrochloric acid is a strong acid, so it does fully dissociate into ions and is a good conductor.
It is a strong acid of 1 mol dm−3 concentration so will have a pH of 0 (−log101 = 0) not a pH greater than 7.
Therefore 1 and 3 only is the correct answer.
Which of the following are true statements about a 1 mol dm−3 aqueous solution of ammonia?
1: It fully dissociates into ions in aqueous solution
2: It will have a pH greater than 7
3: Dissociation should be represented as: NH3(aq) + H2O(l) → NH4+(aq) + OH–(aq)
Strong acids and alkalis fully dissociate into ions in aqueous solution, weak acids and alkalis only partially dissociate. Strong acids and alkalis will react faster than weak acids and alkalis, and will have greater electrical conductivity.
Ammonia solution is a weak alkali so it does not fully dissociate into ions.
It will have a pH greater than 7 as it is alkaline.
Dissociation should not be represented using → (as this suggests complete dissociation) but should be represented using ⇌ (an equilibrium sign). The equation is overwise correct however.
Therefore 2 only is the correct answer.
Which of the following are true statements about hydrochloric acid and ethanoic acid of equal concentration and volume?
1: The hydrochloric acid will react more quickly (faster rate of bubbles) with magnesium ribbon
2: The pH of hydrochloric acid will be lower than that of ethanoic acid
3: The number of moles of hydrochloric acid and ethanoic acid will be the same
Strong acids and alkalis fully dissociate into ions in aqueous solution, weak acids and alkalis only partially dissociate. Strong acids and alkalis will react faster than weak acids and alkalis, and will have greater electrical conductivity.
Hydrochloric acid is a strong acid and ethanoic acid is a weak acid.
The pH of hydrochloric acid will be lower than that of ethanoic acid as at any moment in time there will be a higher concentration of H+ ions (as HCl fully dissociates).
The acids have equal concentration of the acid (but not of H+ ions in solution) and equal volumes, so the number of moles of the acids will be the same. (Moles = Concentration × Volume)
Therefore 1, 2 and 3 is the correct answer.
Which of the following gases, when dissolved in rainwater, are responsible for acid deposition?
1: nitrogen dioxide
2: sulfur dioxide
3: carbon dioxide
Rain water is naturally acidic due to dissolved carbon dioxide, but this only causes mild acidity (pH ≈ 5.6).
Acid deposition occurs when oxides of nitrogen and sulfur dissolve in rain water.
Therefore 1 and 2 only is the correct answer.
Which of the following methods are effective in preventing acid deposition?
1: Filtering waste gases
2: Spraying waste gases with a limestone slurry
3: Passing waste gases through a catalytic converter
Acid deposition occurs when oxides of nitrogen and sulfur dissolve in rain water.
Acid deposition can be prevented by spraying waste gases with a limestone slurry (wet scrubbing in factory chimneys for example) or by passing waste gases through a catalytic converter (in cars for example).
Filtering will not remove gases.
Therefore 2 and 3 only is the correct answer.
Paper 1
Core (SL&HL): Acids and Bases core (SL and HL) paper 1 questions
AHL (HL only): Acids and Bases AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Acids and Bases core (SL & HL) paper 2 questions
AHL (HL only): Acids and Bases AHL (HL only) paper 2 questions
How much of pH, strong and weak acids and acid deposition have you understood?












 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn