 This section is focused on calculations, so there is not a lot of information to learn. However, weak acid and base calculations always crop up in the exam paper somewhere, so it is important to understand the relationships between the variables and the constants in this topic and to ensure practice in the calculations.
This section is focused on calculations, so there is not a lot of information to learn. However, weak acid and base calculations always crop up in the exam paper somewhere, so it is important to understand the relationships between the variables and the constants in this topic and to ensure practice in the calculations.
Ensure you are confident using the terms below and learn the asterisked* definitions
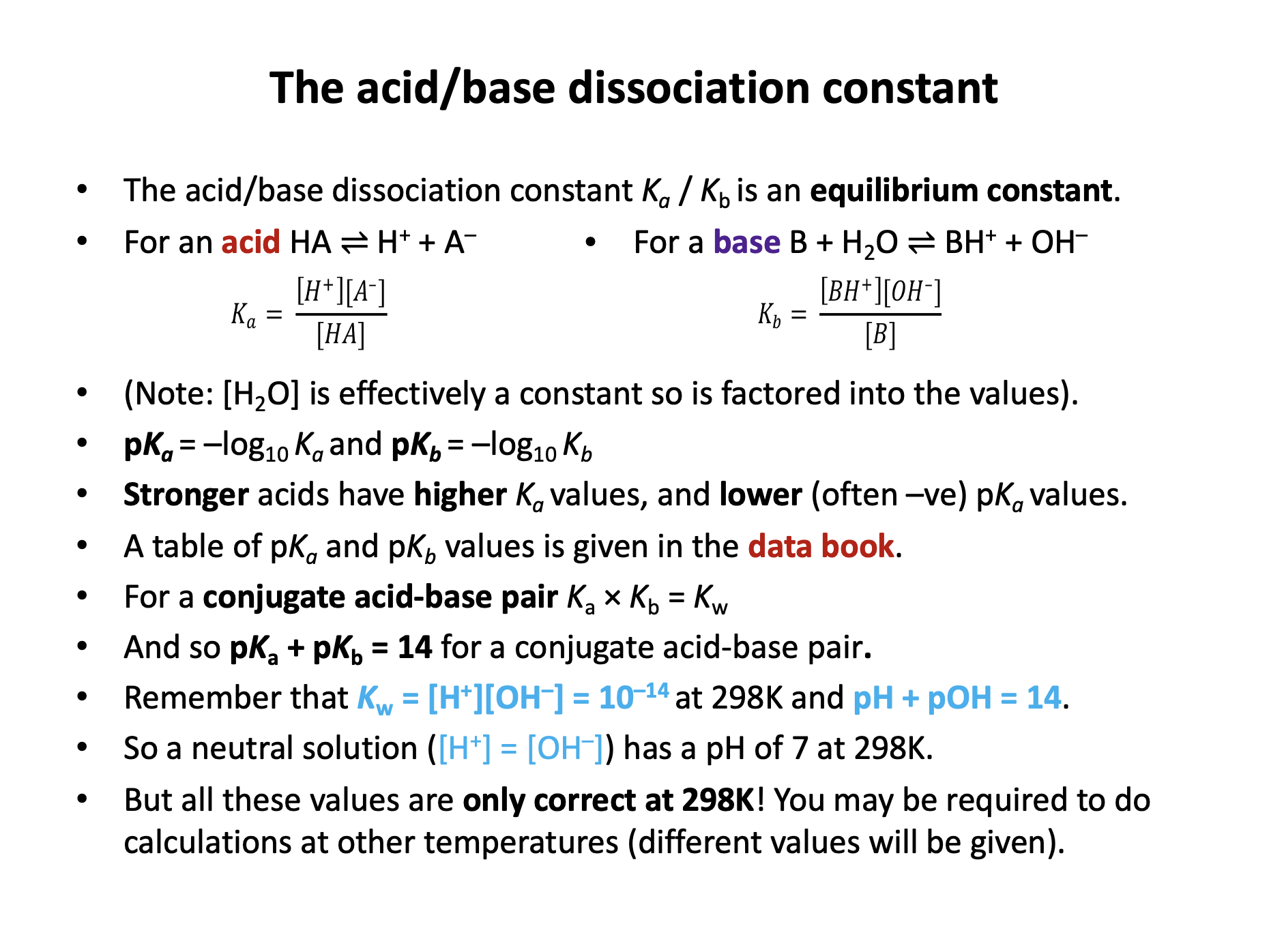
Acid dissociation constant Ka*, Base dissociation constant, Kb*, pKa / pKb*
Which is the correct acid dissociation expression for the aqueous equilibrium as given below?
CH3CO2H ⇌ CH3CO2− + H+
Any equilibrium constant (including the acid dissociation constant) is defined as being the product of the concentrations of the products (each to the power of the number of moles) over the product of the concentrations of the reactants (each to the power of the number of moles), e.g. for a reaction:
aA + bB ⇌ cC + dD
![]()
Therefore the correct answer here is:
\(K_a = {{[CH_3CO_2^-][H^+]} \over [CH_3CO_2H]}\)
Which is the correct base dissociation expression for the aqueous equilibrium as given below?
NH3 + H2O ⇌ NH4+ + OH−
Any equilibrium constant (including the base dissociation constant) is defined as being the product of the concentrations of the products (each to the power of the number of moles) over the product of the concentrations of the reactants (each to the power of the number of moles), e.g. for a reaction:
aA + bB ⇌ cC + dD
![]()
However, the concentration of water in an aqueous solution is effectively constant, so [H2O] is incorporated into the constant. Therefore the correct answer here is:
\(K_b = {{[NH_4^+][OH^-]} \over [NH_3]}\)
Which of the following are true statements about 1 mol dm−3 aqueous solutions of hydrochloric acid and ethanoic acid?
1: The Ka of hydrochloric acid is greater than that of ethanoic acid.
2: The pKa of hydrochloric acid is greater than that of ethanoic acid.
3: The pH of hydrochloric acid is greater than that of ethanoic acid.
Strong acids (like hydrochloric) and alkalis fully dissociate into ions in aqueous solution, weak acids (like ethanoic) and alkalis only partially dissociate.
\(K_a = {{ [A^-][H^+] } \over [HA]}\)
Therefore the Ka for a strong acid will be greater than for a weak acid (more ions on top of fraction).
pKa = −log10Ka
Therefore higher Ka leads to lower pKa (similarly to the relationship between [H+] and the pH scale).
Strong acids will always have lower pH than weak acids of the same concentration, again because of the dissociation.
Therefore 1 only is the correct answer.
Which of the following are true statements about 1 mol dm−3 aqueous solutions of sodium hydroxide and ammonia?
1: The Kb of sodium hydroxide is greater than that of ammonia.
2: The pKb of sodium hydroxide is greater than that of ammonia.
3: The pH of sodium hydroxide is greater than that of ammonia.
Strong acids and bases (like sodium hydroxide) fully dissociate into ions in aqueous solution, weak acids and bases (like ammonia) only partially dissociate.
\(K_b = {{ [OH^-][BH^+] } \over [B]}\)
Therefore the Kb for a strong base will be greater than for a weak base (more ions on top of fraction).
pKb = −log10Kb
Therefore higher Kb leads to lower pKb (similarly to the relationship between [H+] and the pH scale).
Strong bases will always have higher pH than weak bases of the same concentration, again because of the dissociation.
Therefore 1 and 3 only is the correct answer.
The Ka for methanoic acid (HCO2H) at 298K is 1.78 × 10−4. What is the pKb of the methanoate ion (HCO2−) at 298K (3sf)?
Ka = 1.78 × 10−4 (given in question).
pKa = −log10Ka = −log101.78 × 10−4 = 3.74958
For any given conjugate acid-base pair at 298K pKa + pKb = 14. The methanoate ion is the conjugate base of methanoic acid.
Therefore, methanoate ion pKb = 14 − 3.74958 = 10.25042 = 10.3 (3sf)
10.3 is thus the corect answer.
(This can also be calculated using Ka × Kb = Kw)
Incorrect answers
17.8 is a missed negative sign in the pKa expression.
10.2 is a rounding error (a 5 rounds up by convention).
17.7 assumes a missed negative sign in the pKa expression and includes a rounding error (a 5 rounds up by convention).
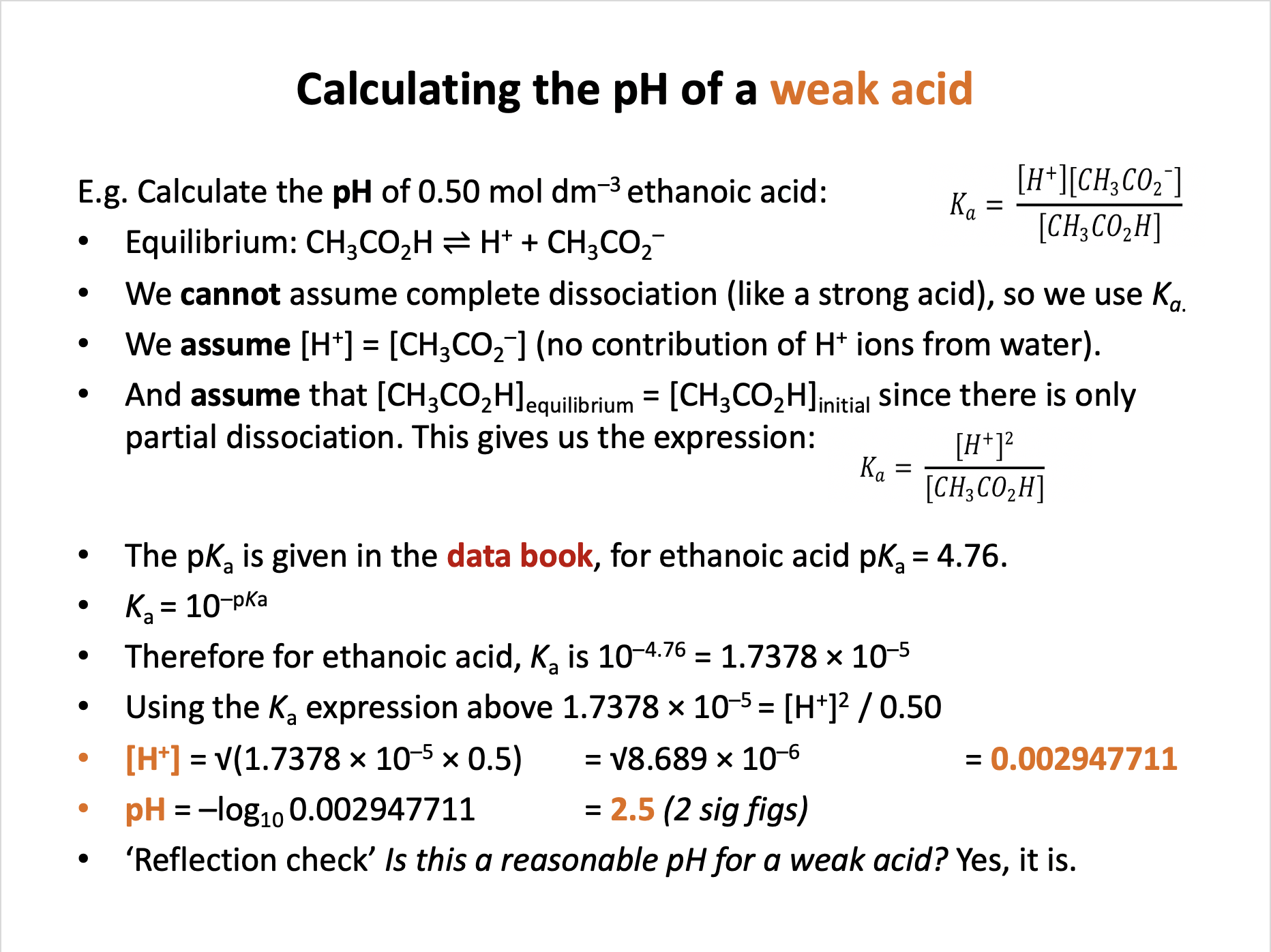
Which of the following are assumptions that we make when calculating the pH of ethanoic acid?
1: That [CH3CO2H] = [CH3CO2−]
2: That water makes no contribution to [H+] at equilibrium
3: That the concentration of the undissociated acid at equilibrium is the same as the initial concentration
For a weak acid: HA ⇌ H+ + A–
The assumptions that we make when calculating the pH of weak acids (like ethanoic) include:
We assume [H+] = [A–] and therefore that there is no contribution by water to [H+].
And we assume that [CH3CO2H] at equilibrium = initial [CH3CO2H] since there is only partial dissociation.
This gives us the expression:
\(K_a = {{ [H^+]^2 } \over [HA]}\)
We do not assume that [CH3CO2H] = [CH3CO2−].
Therefore 2 and 3 only is the correct answer.
What is the pH of a 0.20 mol dm−3 aqueous solution of methanoic acid at 298K (2sf)?
pKa for methanoic acid = 3.75 at 298K (from the data book)
For a weak acid: HA ⇌ H+ + A–
The assumptions that we make when calculating the pH of weak acids include:
We assume [H+] = [A–] and therefore that there is no contribution by water to [H+].
And we assume that [HA] at equilibrium = initial [HA] since there is only partial dissociation.
This gives us the expression:
\(K_a = {{ [H^+]^2 } \over [HA]}\)
Using the pKa given, Ka = 10–pKa = 10−3.75 = 1.77828 × 10−4
And using the equation one row above: 1.77828 × 10−4 = [H+]2 / 0.20
[H+]2 = 1.77828 × 10−4 × 0.20 = 3.5565 × 10−5
[H+] = 0.0059637
pH = −log10 0.0059637 = 2.224485 = 2.2 (2sf)
Therefore 2.2 is the correct answer.
Incorrect answers
4.4 is forgetting the square root
0.062 is using pKa (3.75) in the Ka expression instead of Ka (1.77828 × 10−4)
0.70 assumes a strong acid, so pH = −log10 0.20
What is the pH of a 0.50 mol dm−3 aqueous solution of propanoic acid at 298K (2sf)?
Ka for propanoic acid = 1.35 × 10−5 at 298K
For a weak acid: HA ⇌ H+ + A–
The assumptions that we make when calculating the pH of weak acids include:
We assume [H+] = [A–] and therefore that there is no contribution by water to [H+].
And we assume that [HA] at equilibrium = initial [HA] since there is only partial dissociation.
This gives us the expression:
\(K_a = {{ [H^+]^2 } \over [HA]}\)
1.35 × 10−5 = [H+]2 / 0.50
[H+]2 = 1.35 × 10−5 × 0.50 = 6.75 × 10−6
[H+] = 0.0025981
pH = −log10 0.0025981 = 2.5853481 = 2.6 (2sf)
Therefore 2.6 is the correct answer.
Incorrect answers
5.2 is forgetting the square root
−2.6 is forgetting the minus in the pH expression
−5.2 is forgetting the square root and the minus in the pH expression
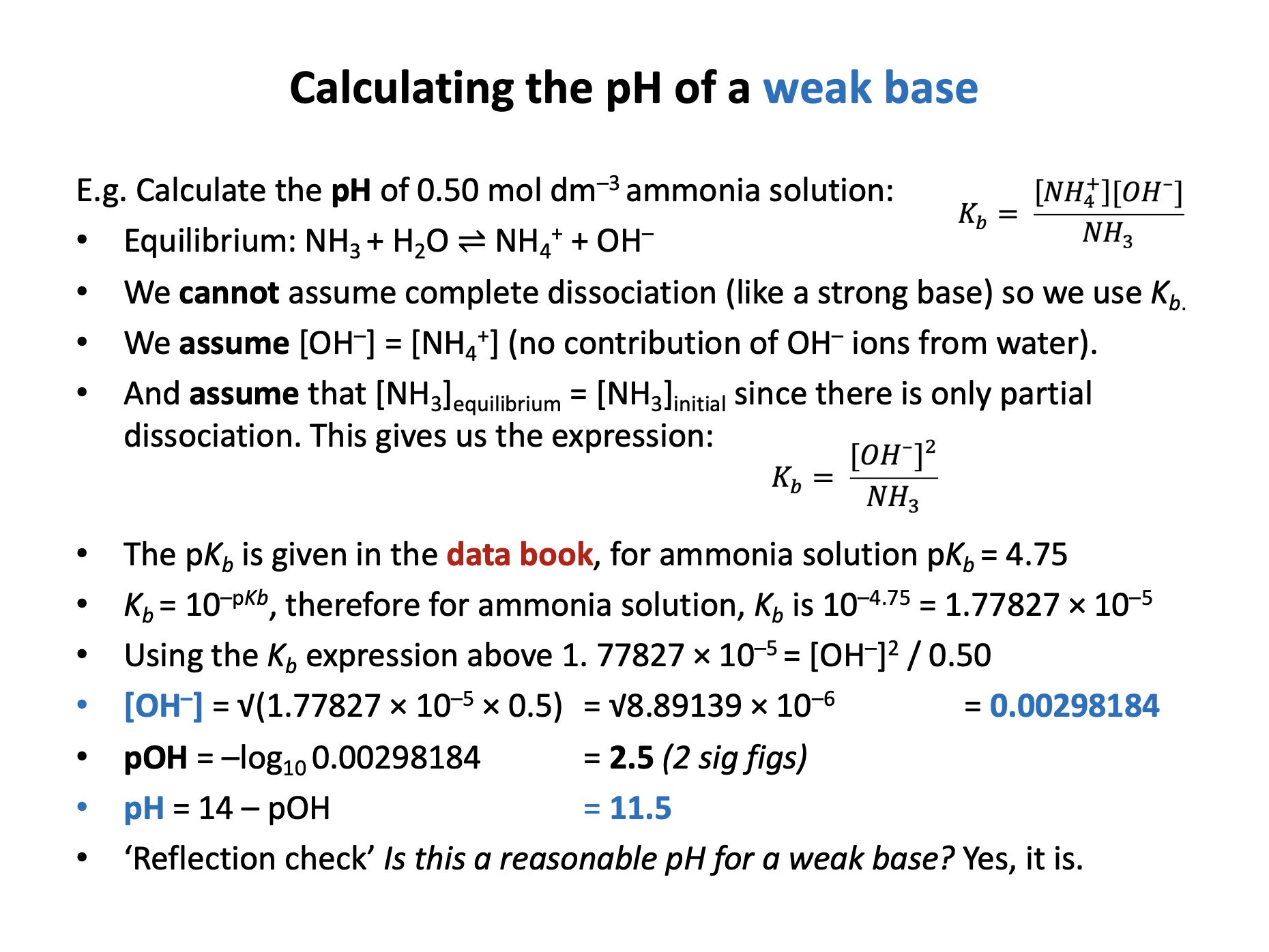
What is the pH of a 0.50 mol dm−3 aqueous solution of methylamine (CH3NH2) at 298K (3sf)?
Kb for methylamine = 4.57 × 10−4 at 298K
For a weak base: B + H2O ⇌ BH+ + OH–
The assumptions that we make when calculating the pH of weak bases include:
We assume [OH−] = [BH+] and therefore that there is no contribution by water to [OH−].
And we assume that [B] at equilibrium = initial [B] since there is only partial dissociation.
This gives us the expression:
\(K_b = {{ [OH^-]^2 } \over [B]}\)
4.57 × 10−4 = [OH−]2 / 0.50
[OH−]2 = 4.57 × 10−4 × 0.50 = 2.285 × 10−4
[OH−] = 0.0151162
pOH = −log10 0.0151162 = 1.8205569
pH = 14−1.8205569 = 12.179443 = 12.2 (2sf)
Therefore 12.2 is the correct answer.
Incorrect answers
10.4 is forgetting the square root
1.82 is giving the pOH instead of pH
3.64 is forgetting the square root and giving the pOH instead of pH
Paper 1
Core (SL&HL): Acids and Bases core (SL and HL) paper 1 questions
AHL (HL only): Acids and Bases AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Acids and Bases core (SL & HL) paper 2 questions
AHL (HL only): Acids and Bases AHL (HL only) paper 2 questions
How much of Calculations involving acids and bases have you understood?








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn