 The photoelectric effect is indisputable evidence for the particle nature of light. Particles of light are discrete and named photons. The energy of a photon depends not on intensity but frequency.
The photoelectric effect is indisputable evidence for the particle nature of light. Particles of light are discrete and named photons. The energy of a photon depends not on intensity but frequency.
Key Concepts
There is a significant body of evidence for the wave model of light including interference and diffraction. Reflection and refraction are also wave properties, but are not exclusive to waves.
The photoelectric effect is evidence only for the quantum nature of light.
When light is shone on a metal plate, electrons in the plate are excited and may escape the surface (photoelectrons). The results of the experiment are:
- Below a certain frequency of light, no electrons are emitted, regardless of the intensity of the light. This is evidence against the wave theory (in which continuous waves would eventually build up sufficient energy for escape).
- When the frequency is increased (i.e. towards or into the ultraviolet region of the electromagnetic spectrum), a threshold frequency is reached at which electrons are emitted. These are the electrons closest to the surface of the metal. The energy required to just release the electrons is the work function. This observation indicates that a single particle of light gives its entire energy to a single electron.
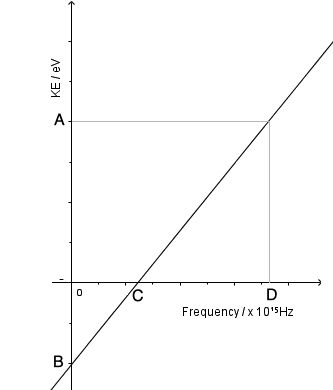
- Above the threshold frequency, increasing the frequency gives the electrons a higher maximum kinetic energy. The range of kinetic energies comes from the variation in where the electrons were located in the metal. This graph shows how the energy of the photoelectrons varies with frequency; none are emitted before the threshold frequency but then kinetic energy increases linearly with frequency.
- Above the threshold frequency, increasing the intensity increases the number of electrons released in a given time, but not the maximum kinetic energy.
The main deficiency in the wave model of light for explaining the photoelectric effect is that an increased intensity should increase the energy of the electrons. Over time, enough energy would be built up for electrons to be released. And the intensity should increase the maximum kinetic energy of the electrons.
How much of Photoelectric effect and photons have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn