 Radioactive decay is a random process. This leads to an interesting link with mathematics.
Radioactive decay is a random process. This leads to an interesting link with mathematics.
If the rate of change of a quantity is proportional to the quantity itself then the change is exponential.
Key Concepts
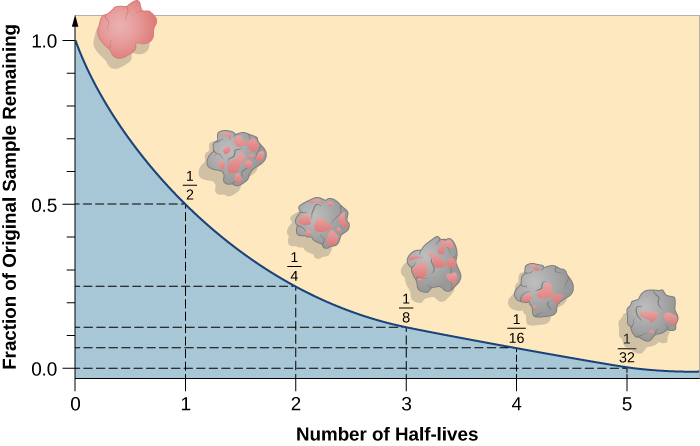
Nuclear decay is a random process. In a sample of unstable nuclei:
- We do not know which nucleus will be the next to decay
- We do not know when the next nucleus will decay
- There is a fixed probability that a given proportion of decays will occur in a given period of time. For a large sample of dice, one sixth would land on a six in a given roll.
The probability of decay is related to the amount of energy that will be released when it happens.
The consequence of the random nature of decay can be demonstrated with a more everyday substance: beer foam. Since the bubbles pop randomly, the amount of beer foam in a glass decays exponentially.
Exponential decay graphs are an example of a Non-linear graph.
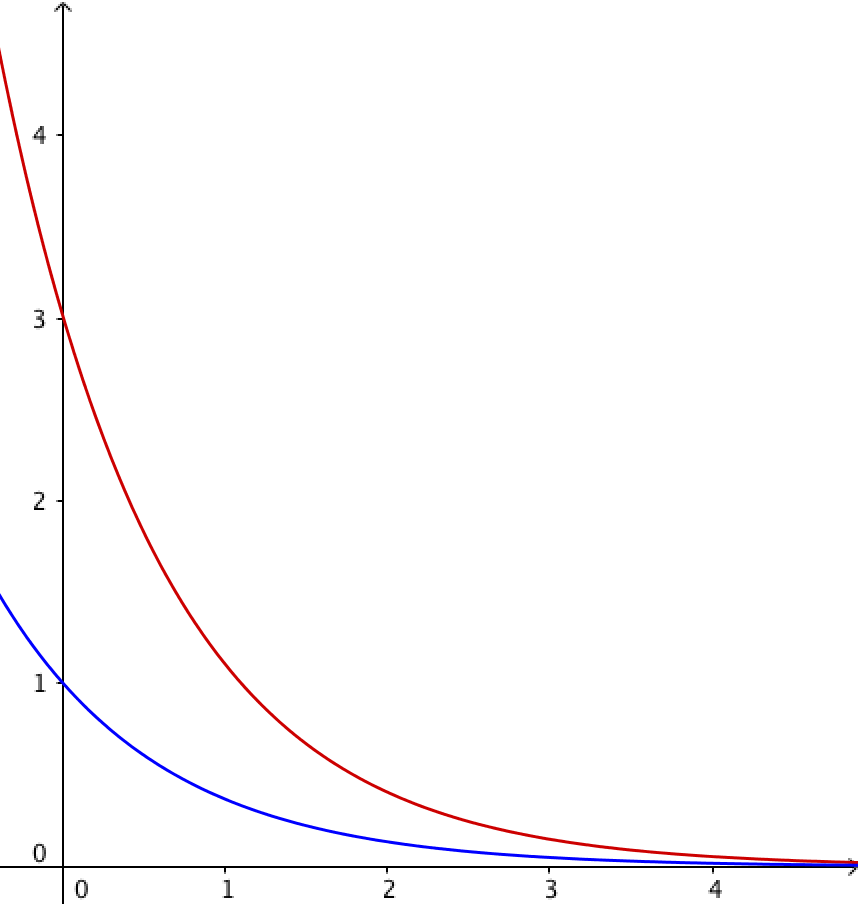
Exponential decay
If \({\mathrm{d} N\over \mathrm{d}t} ∝ N\) then the decay is exponential:
\(N =N_0\mathrm{e}^{-\lambda t}\)
- \(N\) is the number of undecayed nuclei remaining
- \(N_0\) is the number of undecayed nuclei when \(t=0\)
- \(\lambda\) is the decay constant, a fixed probability of a given proportion of nuclei decaying per unit time (units correspond to \(t\) e.g. s-1)
- \(t\) is time (e.g. s)
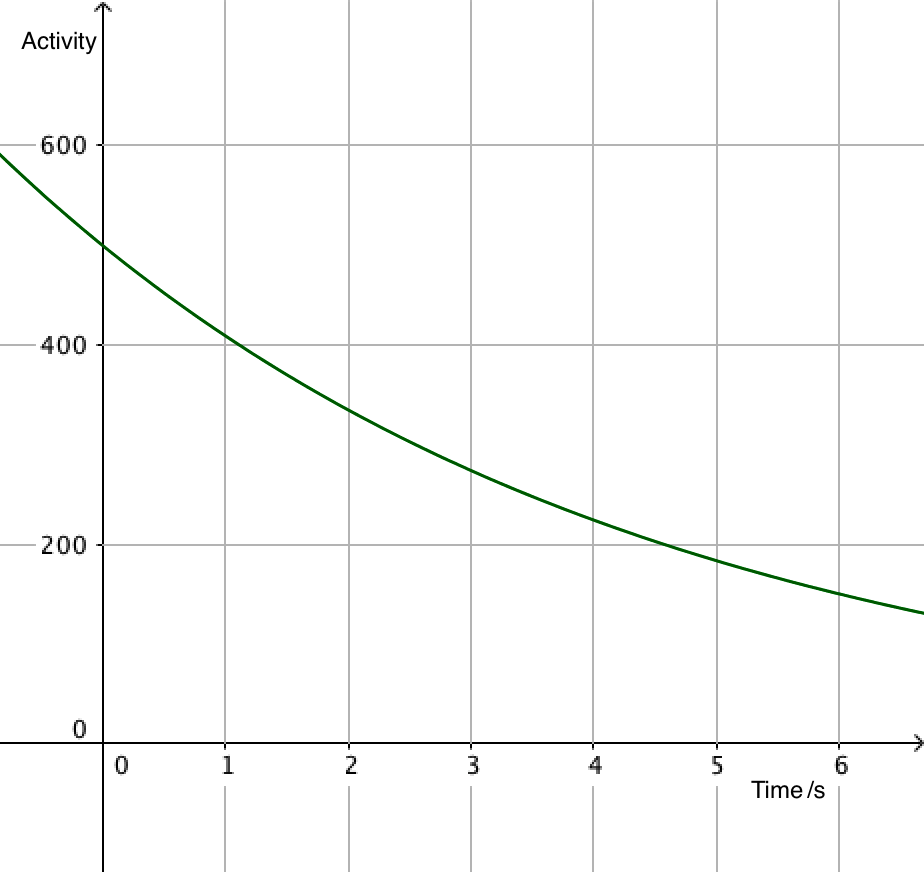
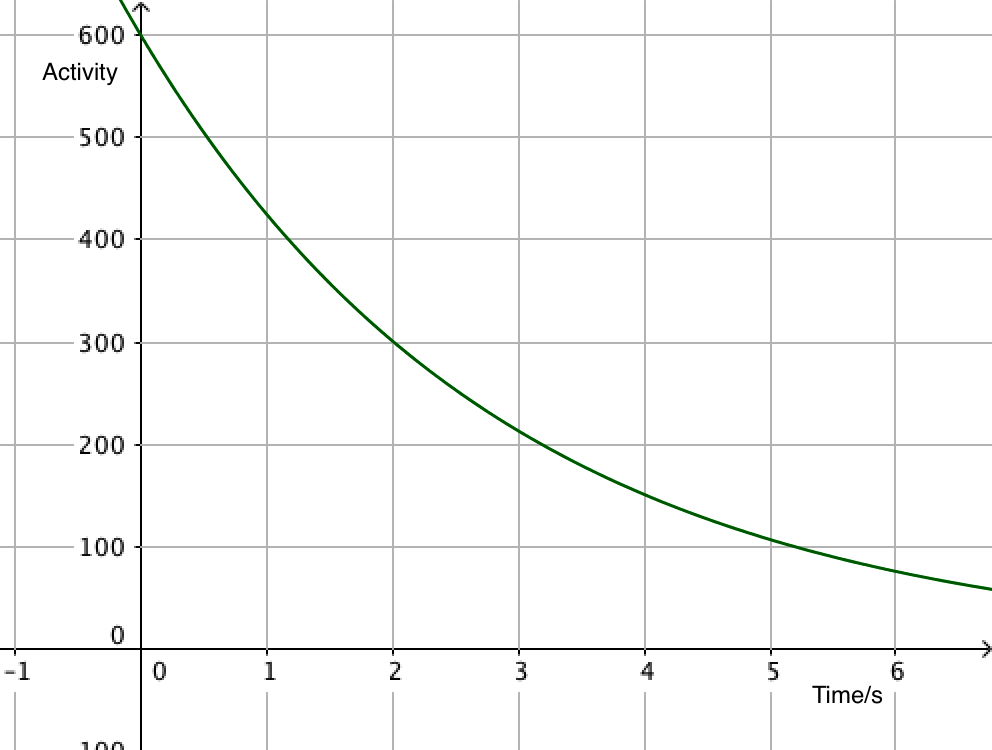
Since activity is proportional to the number of undecayed nuclei, it is also exponential, leading to equations that can often be more useful than the number of particles. After all, it's difficult to count individual nuclei!
\(A=\lambda N_0\mathrm{e}^{-\lambda t}\)
\(A =A_0\mathrm{e}^{-\lambda t}\)
- \(A\) is the activity of the source (s-1)
- \(A_0\) is the activity when \(t=0\)
The exponential decay equation enables the calculation of the number of nuclei remaining at any time.
A linear relationship can be produced by taking natural logarithms of each side of the equation:
\(\ln A=-\lambda t+\ln{A_0}\)
This is the kind of mathematical manipulation that may required for your Investigation.
How much of AHL Nuclear decay have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn