 The structure of matter can be investigated using scattering experiments, both of whole nuclei and of electrons.
The structure of matter can be investigated using scattering experiments, both of whole nuclei and of electrons.
Knowledge of the radius of one nuclear isotope can be used to calculate the radius of any other.
Key Concepts
The Rutherford scattering experiment can be used to determine the radius of a nucleus.
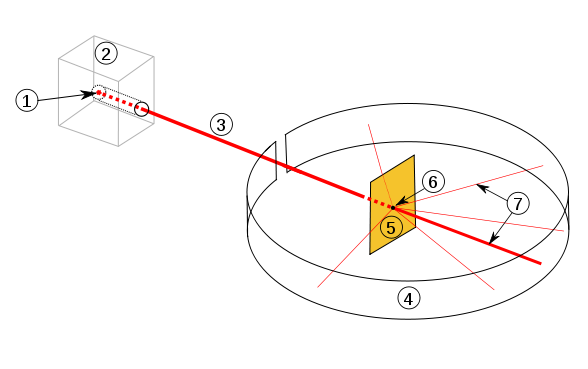
As the angle is increased from the initial beam direction, the number of alpha particles decreases. This is due to the repulsive elecric forces between the alpha particles and the nucleus, which increases in magnitude with shorter distances. The scattering distribution can be predicted by an equation devised by Rutherford (not required for the course).
In high energy scattering experiments, the alpha particles move close enough to the nucleus to be within the range of the strong nuclear force. This means that the scattering distribution is affected. The point at which the Rutherford scattering equation no longer applies can be used to find the effective nuclear radius.
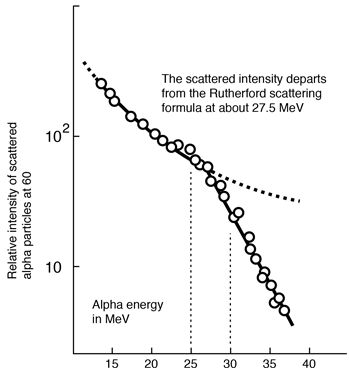
Eisberg, R. M. and Porter, C. E., Rev. Mod. Phys. 33, 190 (1961)
The strong nuclear force is short range; unlike gravitation, the magnitude of force does not vary as an inverse square law. Nuclear density is approximately the same for all nuclei. The consequence is that the radius of any nucleus is related only to the number of nucleons:
\(R=R_0A^{1\over 3}\)
- \(R\) is the radius of a nucleus (m)
- \(R_0\) is a constant (m)
- \(A\) is the number of nucleons
The only macroscopic objects with the same density as nuclei are neutron stars.
How much of AHL Nuclear radius have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn