 Present-day nuclear power relies on nuclear fission - that energy is released when a large, relatively unstable, nucleus splits into two, more stable nuclei. This is a consequence of binding energy in the nucleus. This energy can be used to heat water to produce high pressure steam, and the Power stations process continues from there.
Present-day nuclear power relies on nuclear fission - that energy is released when a large, relatively unstable, nucleus splits into two, more stable nuclei. This is a consequence of binding energy in the nucleus. This energy can be used to heat water to produce high pressure steam, and the Power stations process continues from there.
Pay attention to all of the key words on this page - fuel rods, moderator, critical mass, chain reaction, control rods and coolant.
Key Concepts
Fuel rods (e.g. uranium)
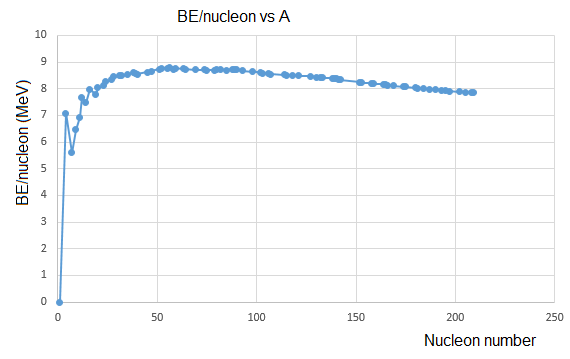
Binding energy is the energy stored when nucleons (protons and neutrons) are held together in the nucleus by the strong nuclear force. As the number of nucleons increases, so does the total binding energy.
However, if we instead consider the binding energy per nucleon (i.e. total divided by the number), we can observe a much more interesting graph when plotted against number of nucleons. The curve below shows that iron-56 is the most stable nucleus, and that larger nuclei will release energy when split.
Naturally occurring uranium contains less than 1% 235U, the rest is other isotopes such as 238U.
For an ongoing fission reaction to take placed, the percentage of 235U needs to be about 3%. We call these fuel rods enriched.
Moderator (e.g. water or graphite)
Fission does not occur without stimulation. Instead, 235U splits when the nucleus absorbs another neutron. Each fission reaction releases 2-3 more nuclei and so the process can continue.
If the neutrons travel too fast they will pass right through the 235U nuclei. To be absorbed the neutrons should have a kinetic energy of about 1 eV, but the neutrons produced in fission have about 1 MeV. To slow the neutrons a material is added into the reactor in between the fuel rods, this material is called a moderator.
Critical Mass
Once the neutrons have been slowed using a moderator, they still need to come into contact with uranium nuclei in order for fission to continue. If there is not enough uranium in a reactor, most of the neutrons will escape
The minimum amount of material required is called the critical mass.
Chain reaction
The fission of 235U is initiated by adding a neutron. When the nucleus splits more neutrons are produced that can initiate more fissions. This equation shows an example of a possible nuclear fission reaction.
![]()
A chain reaction is a self-sufficient ongoing reaction in which the rate of fission is constant or increasing.

Control rods (e.g. boron)
The ideal situation for a nuclear reactor is to achieve a constant rate of fission reactions. This would mean that the temperature remains constant, and that heat is being generated for other processes. If the critical mass is exceeded or if the rate of reaction needs to decrease, control rods can be lowered into the reactor to absorb neutrons completely. A typical material used is boron, as it has stable nuclei.

In the case of a fault and the control rods can not be moved, the reaction can not be slowed down. This means that the reactor can overheat and the vessel itself can melt ("meltdown"). Once this happens the reactor can not be shut down.
Coolant (e.g. water)
The kinetic energy of the fission products is transferred using a simple heat exchanger. A fluid passes on the other side of a barrier to the reactor at pressure produced by a pump. It increases in temperature, therefore reducing the temperature of the interior. Water is an example of a potential coolant material.
How much of Nuclear power I have you understood?


 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn