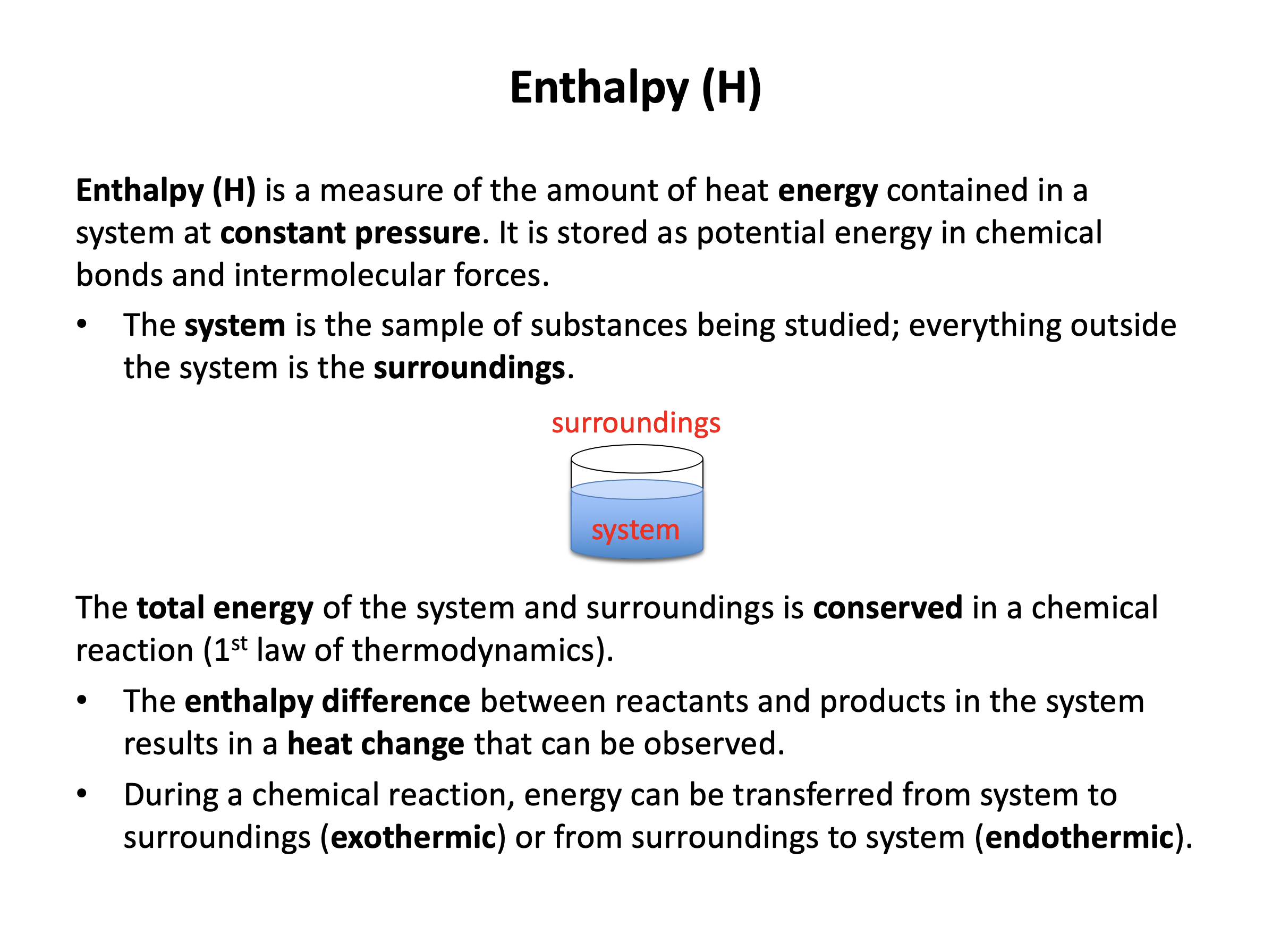
 All physical and chemical processes are accompanied by an energy change. This section explains how the enthalpy change of a chemical reaction can be determined from the effect that it has on the temperature of the surroundings. Use revision cards 6-10 to become familiar with the relevant calorimetry techniques, particularly their limitations. Use the practice questions to ensure that you are confident processing the raw data, writing out the calculations in full to avoid making careless mistakes with units, and stating any assumptions that you make.
All physical and chemical processes are accompanied by an energy change. This section explains how the enthalpy change of a chemical reaction can be determined from the effect that it has on the temperature of the surroundings. Use revision cards 6-10 to become familiar with the relevant calorimetry techniques, particularly their limitations. Use the practice questions to ensure that you are confident processing the raw data, writing out the calculations in full to avoid making careless mistakes with units, and stating any assumptions that you make.
Ensure you are confident using the terms below and learn the asterisked* definitions
enthalpy (H)*, system, surroundings, exothermic, endothermic, standard enthalpy change (ΔHo)*, specific heat capacity
Which of the following are true statements in thermochemistry?
1: Heat is a form of energy.
2: Temperature represents a measure of the average kinetic energy of particles in a system.
3: The energy of a system cannot change.
Heat is a form of energy. It is considered to be a measure of the (kinetic) energy of particles transferred between two systems/system and surroundings.
Temperature represents a measure of the average kinetic energy of particles in a system. Not all the particles will have the same kinetic energy (there will be a distribution of kinetic energies) but temperature represents an average.
The energy of a system can change. Energy can move from one system to another or from a system to surroundings. For example in an exothermic reaction heat energy moves from system to surroundings.
Thus 1 and 2 only is the correct answer.
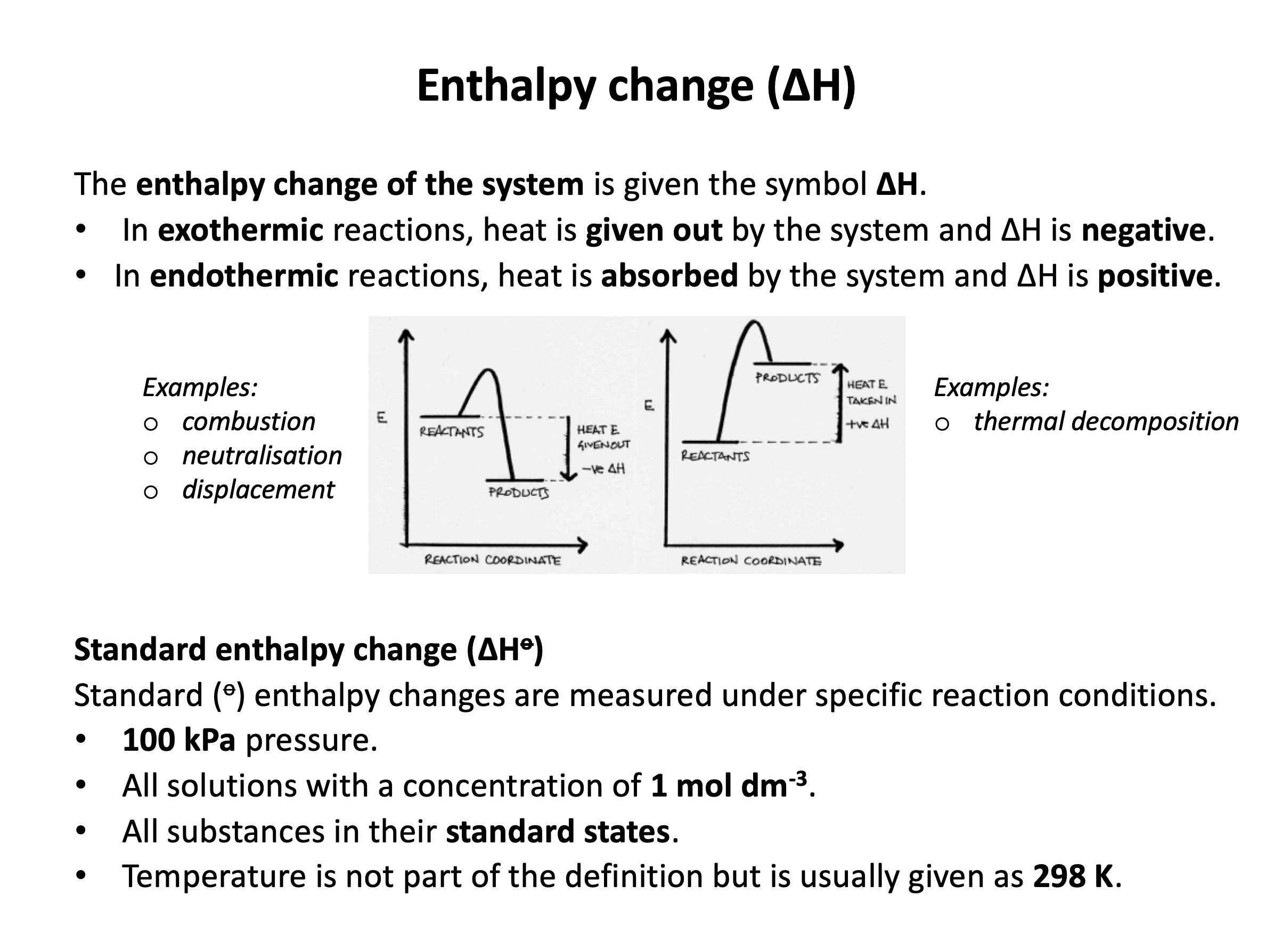
Which of the following best represents 'standard state' in thermochemistry?
Strictly speaking standard states do not include temperature! However, chemists tend to assume a temperature of 298K, so that is included here, and should be included in any answer given.
Standard state does assume a standard pressure of 100kPa (this used to be 1 atmosphere - 101.325kPa - but is now 100kPa). Substances are also assumed to be pure and in the correct state (standard state) at 100kPa (and 298K).
Thus 'pure substance in its standard state at 100kPa and 298K' is the correct answer.
If a chemical reaction is endothermic in the forward direction, which of these statements is true?
If a reaction is endothermic, then heat energy (enthaply) is transferred from the surroundings to the system. Thus the system gains energy, ΔH is positive, and the energy of the products is higher than the energy of the reactants:
On the left; exothermic - on the right; endothermic.
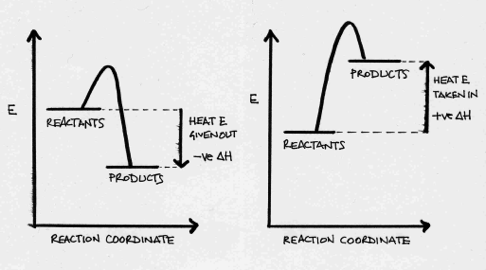
A chemical reaction is carried out in aqueous solution (water), and the temperature of the solution rises by several degrees in temperature. Which of these statements is true with respect to the reaction?
If the temperature of the solution has increased, then the system has released energy to the surroundings. The reaction is therefore exothermic. Thus the system loses energy, ΔH is negative, and the energy of the products is lower than the energy of the reactants:
On the left; exothermic - on the right; endothermic.

Which of the following would be correctly included in the definition of a standard enthalpy of formation?
1: One mole of the compound is formed...
2: ...from its elements...
3: ...in the gaseous state.
Standard enthalpy of formation is a definition that needs to be learned:
Standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their standard states (at 100kPa and 298K).
Thus 1 and 2 only is the correct answer.
Which of the equations below represents the standard enthalpy of combustion of ethane?
Standard enthalpy of combustion is a definition that needs to be learned:
Standard enthalpy of combustion is the enthalpy change when one mole of a compound combusts completely (in excess oxygen) with all reactants and products in their standard states (at 100kPa and 298K).
Thus C2H6(g) + 3½O2(g) → 2CO2(g) + 3H2O(l) is the correct answer.
Errors shown in the other answers include:
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) two moles of ethane (rather than one mole);
C2H6(g) + 3½O2(g) → 2CO2(g) + 3H2O(g) water in the gaseous state (rather than liquid - its standard state);
C2H6(g) + 2½O2(g) → 2CO(g) + 3H2O(l) carbon monoxide produced (rather than carbon dioxide - to show complete combustion).
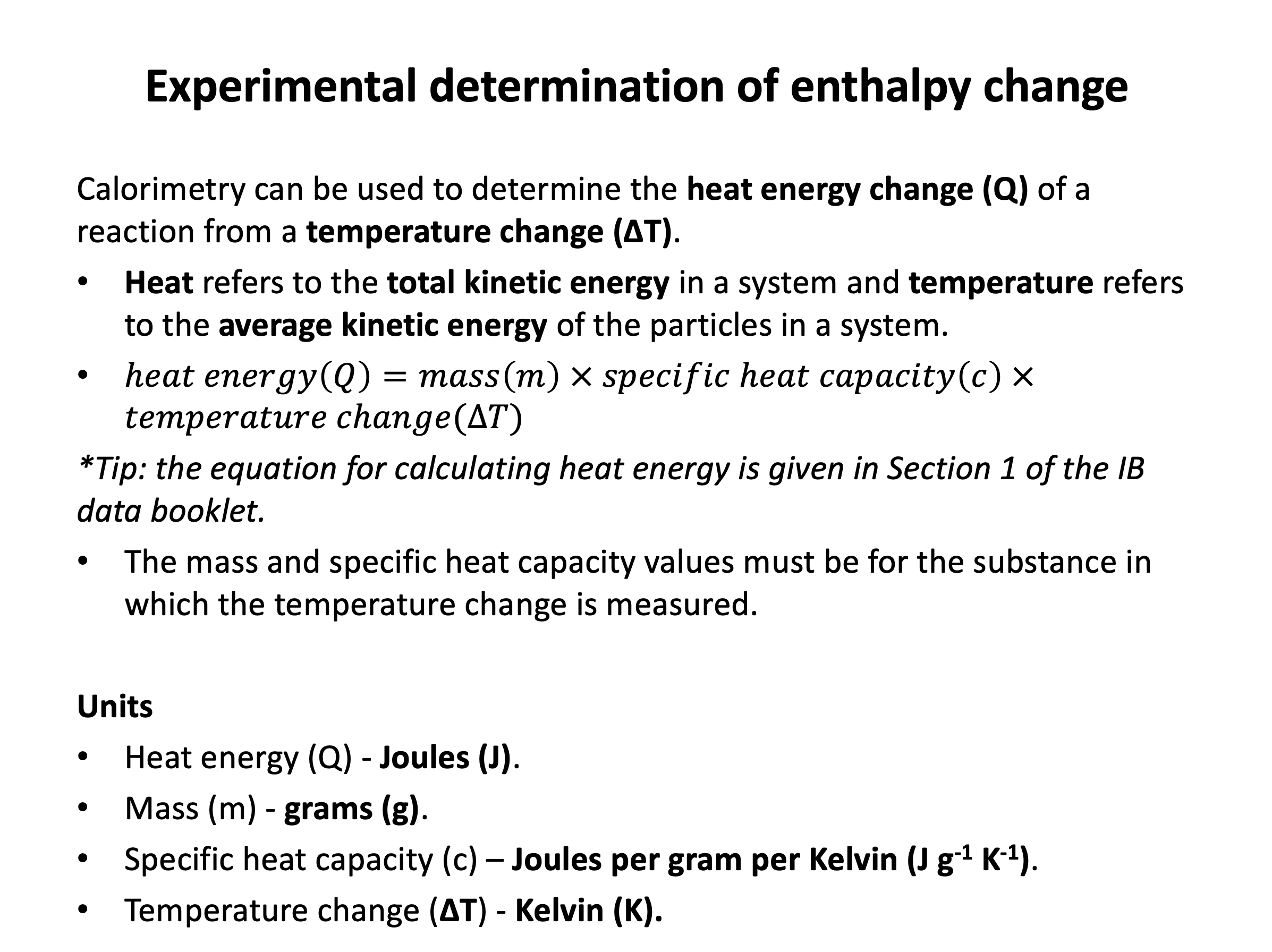
A student investigated a combustion reaction. The student used a spirit burner to burn some fuel and heat 200cm3 of water. During the experiment the water temperature increased from 22.0°C to 38.4°C.
What is the approximate (given to 1 decimal place) amount of heat energy (in kJ) absorbed by the water?
Using Q=mcΔT and a specific heat capacity of water of 4.18 J K−1 g−1 (given in the data book).
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, water. Water has a density of 1 g cm−3 (so 200cm3 is 200g water).
38.4 − 22.0 = 16.4°C (A temperature change of 16.4°C is the same as 16.4K).
Q = 200 × 4.18 × 16.4 = 13710.4J = 13.7 kJ to 1d.p
Thus the answer is 13.7
A student investigated the enthalpy change for solid ammonium chloride (NH4Cl) dissolving in distilled water. The student added 5.59g of ammonium chloride to 100cm3 of water and measured a temperature decrease of 5.0°C.
What is the approximate enthalpy change for this dissolving process for one mole of ammonium chloride (in kJ mol−1)?
Using Q=mcΔT and a specific heat capacity of water of 4.18 J K−1 g−1 (given in the data book).
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, water. Water has a density of 1 g cm−3 (so 100cm3 is 100g water). A temperature change of 5°C is the same as 5K.
Q = 100 × 4.18 × 5 = 2090J
(for the 5.59g of NH4Cl used)
Molar mass of NH4Cl = 14.01 + (1.01×4) + 35.45 = 53.50 g mol−1
Moles of NH4Cl used = mass / molar mass = 5.59 / 53.50 = 0.104485981
For one mole of ammonium chloride the enthalpy change will therefore be:
2090 / 0.104485981 = 20003J mol−1 approximately
Which is 20 kJ mol−1 approximately
And the enthalpy change is endothermic because the temperature dropped, so the sign is positive.
Thus the answer is +20
Assume that the standard enthalpy change of neutralisation for the reaction between hydrochloric acid and sodium hydroxide (one mole of each) is −57.6 kJ mol−1.
If a student adds 500cm3 of 1.00 mol dm−3 HCl(aq) to 500cm3 of 1.00 mol dm−3 of NaOH(aq) and measures the temperature change of the total volume, what might they expect the temperature change to be?
Using Q=mcΔT and a specific heat capacity of water of 4.18 J K−1 g−1 (given in the data book).
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, the solutions are predominently water. Water has a density of 1 g cm−3 (so 1000cm3 is 1000g water).
Moles of HCl (and also NaOH) = concentration (mol dm−3 )× volume (dm3) = 1.00 × 0.500 = 0.500 mol
(Reaction is 1:1 molar ratio)
0.5 of a mole of each are used, therefore the enthalpy change will be:
−57.6 × 0.5 = −28.8 kJ = −28800J
This value is negative, so the reaction is exothermic and the temperature change will be an increase.
Q=mcΔT
28800 = 1000 × 4.18 × ΔT
ΔT = 28800 / (1000 × 4.18) = 6.889952153K
Thus the answer is an increase of 6.9K
A student investigated a combustion reaction. The student used a spirit burner to burn 0.40 mol of fuel and heat 200cm3 of water in a 140g copper calorimeter. During the experiment the temperature of the water and the copper calorimeter increased from 21.4°C to 46.8°C.
The specific heat capacity of copper is 0.385 J K−1 g−1, and for water 4.18 J K−1 g−1 (given in the data book).
What is the approximate enthalpy change of combustion for this fuel (in kJ mol−1)?
Using Q=mcΔT
Q (heat energy) = mass × specific heat capacity × temperature change (these three values are for whatever substance is absorbing the heat energy; in this case, water and also copper. Water has a density of 1 g cm−3 (so 200cm3 is 200g water).
The temperature change (46.8 − 21.4 = 25.4) of 25.4°C is the same as 25.4K.
For the water:
Q = 200 × 4.18 × 25.4 = 21234.4J
And for the copper calorimeter:
Q = 140 × 0.385 × 25.4 = 1369.06J
Total Q = 21234.4 + 1369.06 = 22603.46J
(for the 0.40 mol of fuel burnt)
For one mole of fuel the enthalpy change will therefore be:
22603.46 / 0.40 = 56508.65J mol−1
Which is 56.5 kJ mol−1 approximately
And the enthalpy change is exothermic, so the sign is negative.
Thus the answer is −56.5
(An answer of −53.1 is obtained by using only the heat energy absorbed by the water)
(An answer of −90.3 is obtained by using a mass of 340g of water)
(An asnwer of −9.0 is obtained by multiplying by 0.40 (mol) instead of dividing)
Paper 1
Core (SL&HL): Energetics core (SL and HL) paper 1 questions
AHL (HL only): Energetics AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Energetics core (SL & HL) paper 2 questions
AHL (HL only): Energetics AHL (HL only) paper 2 questions
How much of Measuring energy changes have you understood?














 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn