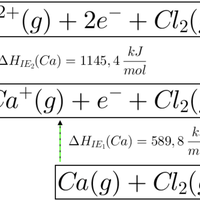 This section involves more complex energy cycles than those you came across in Section 5.2. Born Haber cycles can be used to determine the lattice enthalpies and enthalpies of solution of ionic compounds. Use the flash cards to learn the vocabulary thoroughly, as the definition of an enthalpy changes will determine how the its data in a Born Haber cycle needs to be manipulated. To avoid careless mistakes, always draw a well-spaced and annotated energy cycle – refer to revision cards 5, 6 and 10 to help you organise your thoughts and working.
This section involves more complex energy cycles than those you came across in Section 5.2. Born Haber cycles can be used to determine the lattice enthalpies and enthalpies of solution of ionic compounds. Use the flash cards to learn the vocabulary thoroughly, as the definition of an enthalpy changes will determine how the its data in a Born Haber cycle needs to be manipulated. To avoid careless mistakes, always draw a well-spaced and annotated energy cycle – refer to revision cards 5, 6 and 10 to help you organise your thoughts and working.
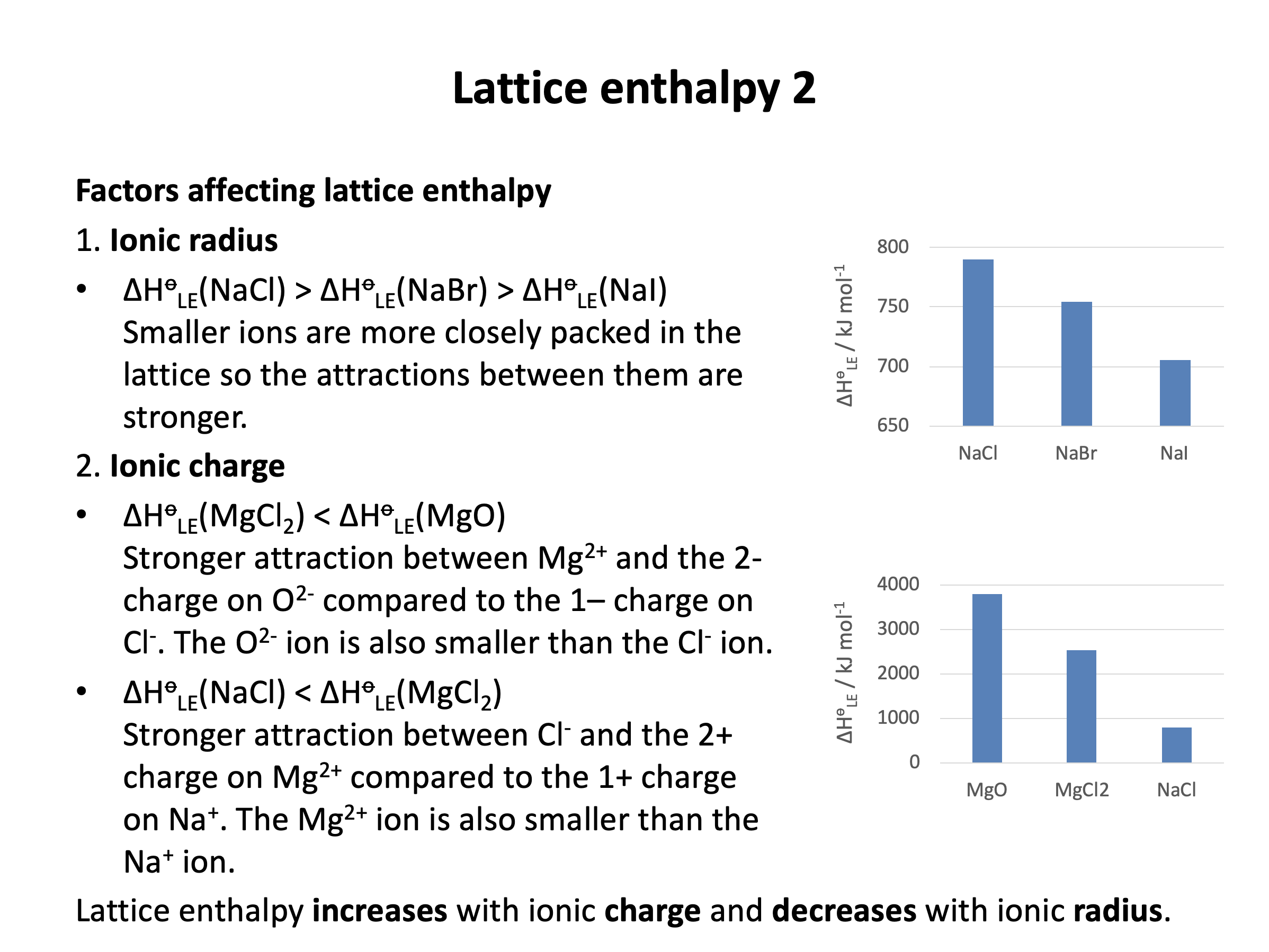
You should also be able to explain how ionic radius and ionic charge affect the lattice enthalpy of an ionic compound.
Ensure you are confident using the terms below and learn the asterisked* definitions
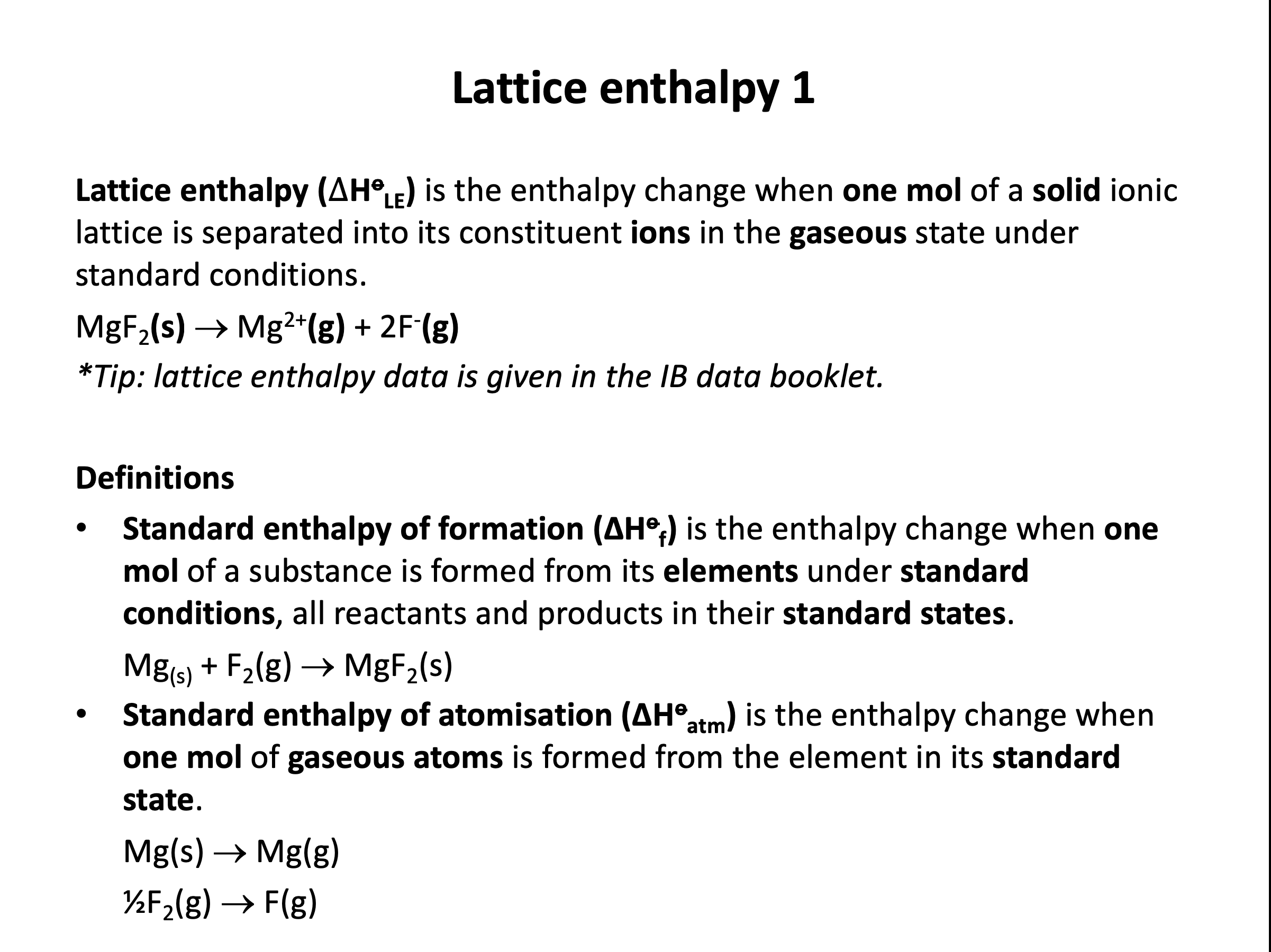
lattice enthalpy (ΔoHLE)*, standard enthalpy of atomisation (ΔoHatm)*, first ionisation enthalpy (1st IE)*, first electron affinity (1st EA)*, enthalpy of solution (ΔHosoln)*, hydration enthalpy (ΔHosoln)*
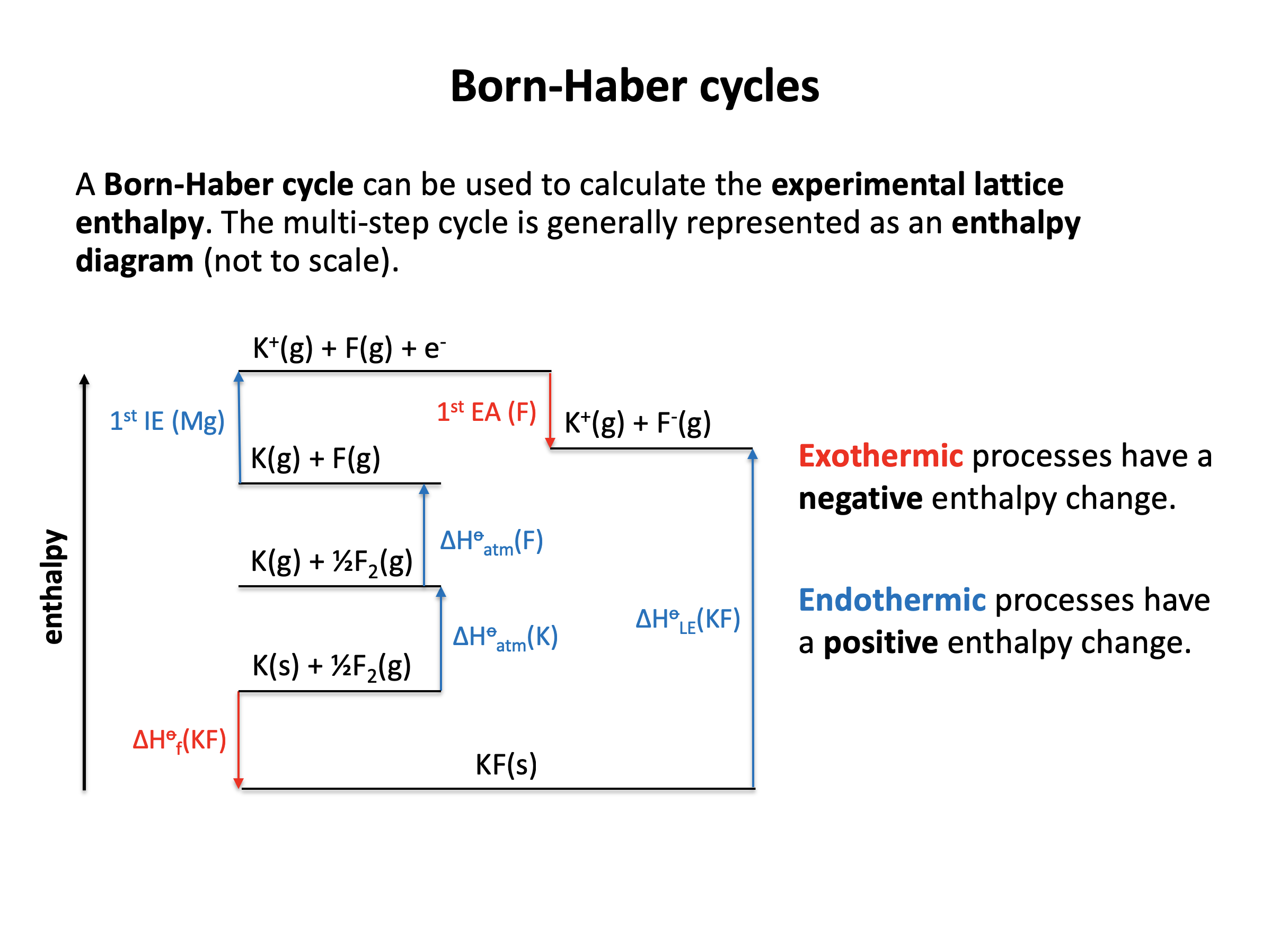
What is the definition of lattice enthalpy (ΔHlatt°)?
The IB definition of lattice enthalpy is usually a positive value (endothermic as ions are pulled apart and bonds are broken), hence:
The enthalpy change that occurs when 1 mol of ionic solid is dissociated into its gaseous ions is the correct answer. e.g. NaCl(s) → Na+(g) + Cl−(g)
Be aware that lattice enthalpy is also often defined as the reverse Na+(g) + Cl−(g) → NaCl(s) in certain text books etc. in which case the sign of the lattice energy value will be negative (exothermic as ions come together and bonds are made).
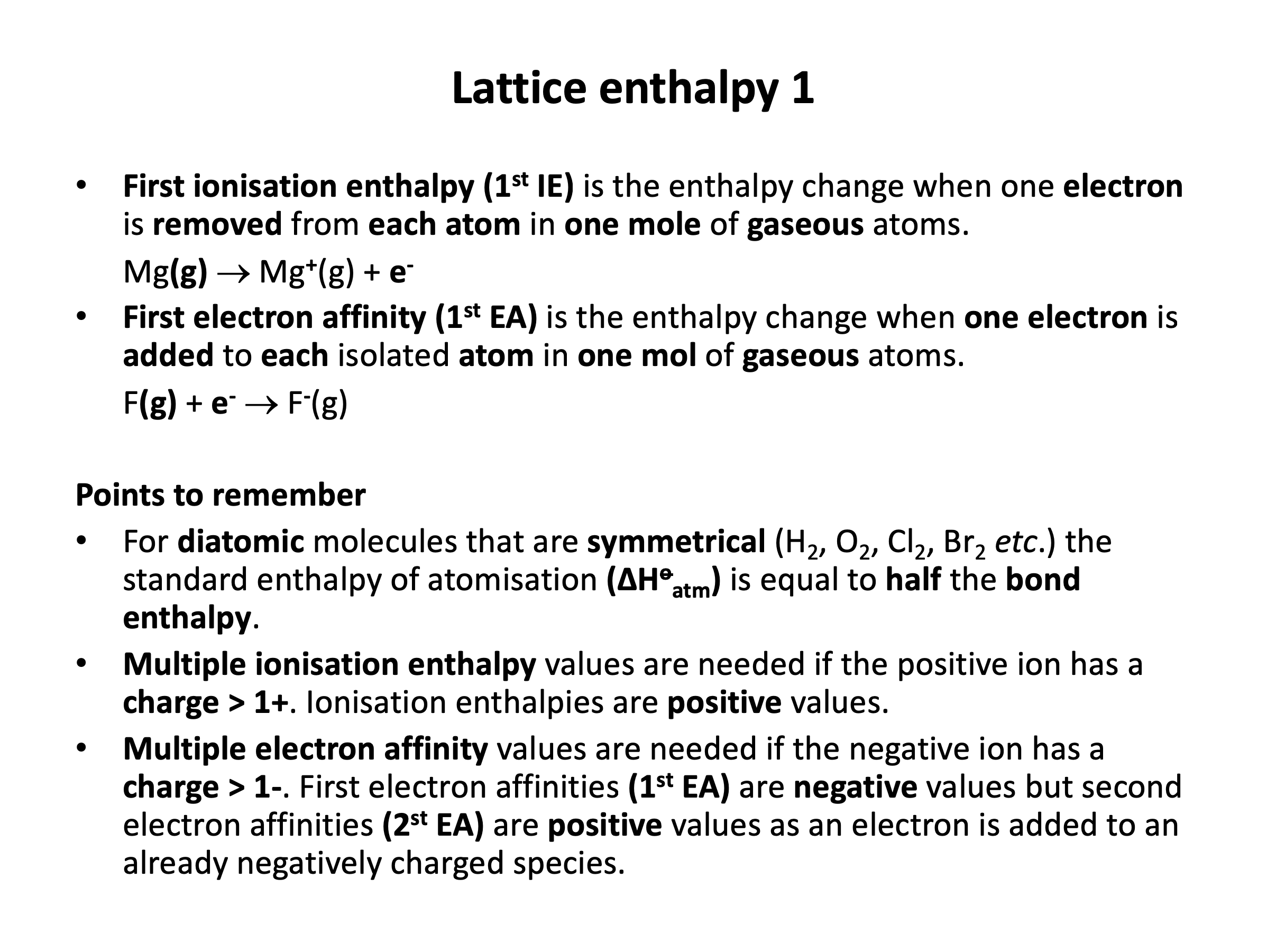
Which of the following would be correctly included in the definition of first ionisation enthalpy?
1: One mole of 1+ ions are formed...
2: ...from the element...
3: ...in its standard state.
First ionisation enthalpy is a definition that needs to be learned:
First ionisation enthalpy is the enthalpy change when one electron is removed from each atom in one mole of gaseous atoms.
Thus 1 only is the correct answer.
One mole of 1+ ions are formed, but first ionisation enthalpy involves gaseous atoms (not the element in its standard state)
Which statement correctly describes the relationship between the lattice enthalpies of sodium chloride (NaCl) and potassium chloride (KCl)?
Lattice enthalpies increase with increasing ionic charge and decrease with increasing ionic radius.
Smaller ions can pack in the lattice more tightly (and/or have higher charge density) and therefore tend to generate stronger attraction in the lattice.
Ions with greater charges tend to generate stonger attraction in the lattice.
The sodium ion is smaller than the potassium ion (sodium is higher in group 1 on the periodic table) and so: NaCl has a larger lattice enthalpy than KCl because the sodium ion is smaller than the potassium ion so there is stronger attraction in the lattice is the correct answer.
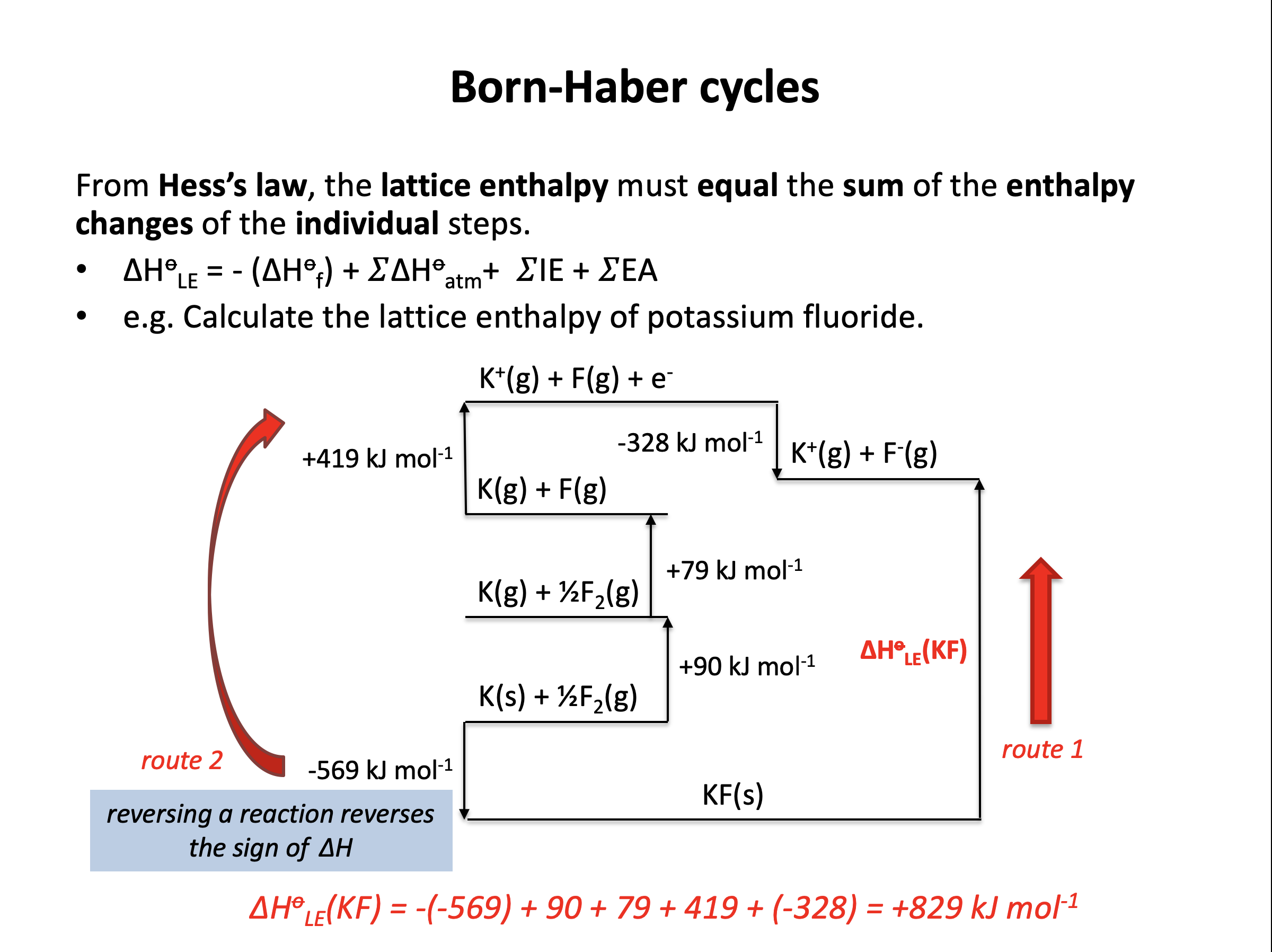
Which of the enthalpy changes given is represented by the letter C on the Born Haber cycle diagram below:
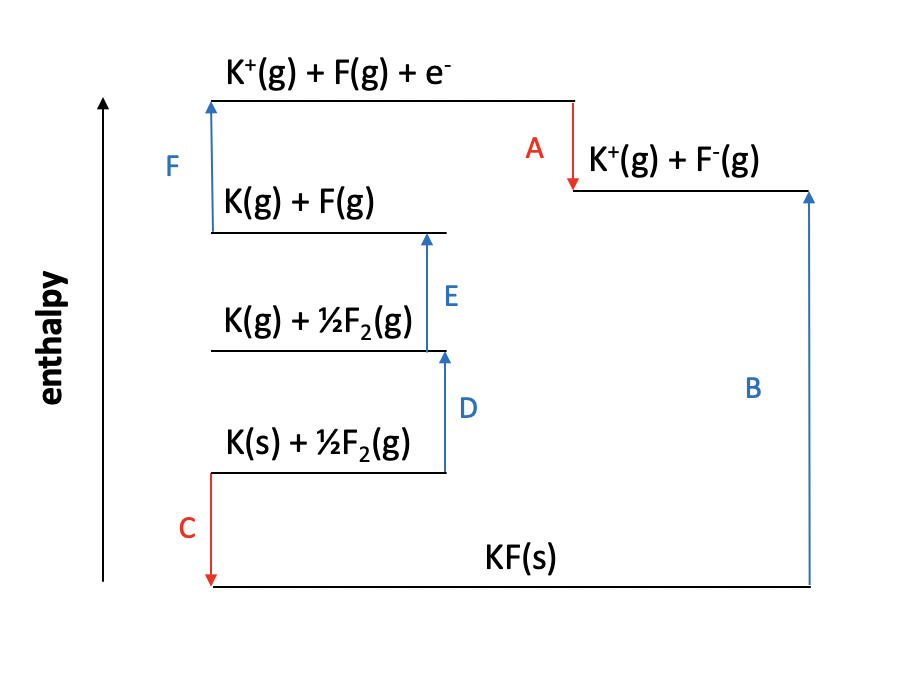
The enthalpy change C on the diagram shows the reaction K(s) + ½F2(g) → KF(s). This is the formation of one mole of a compound from its elements in their standard states.
The correct answer is therefore Enthalpy change of formation for potassium fluoride.
The enthalpy change definitions all need to be learned.
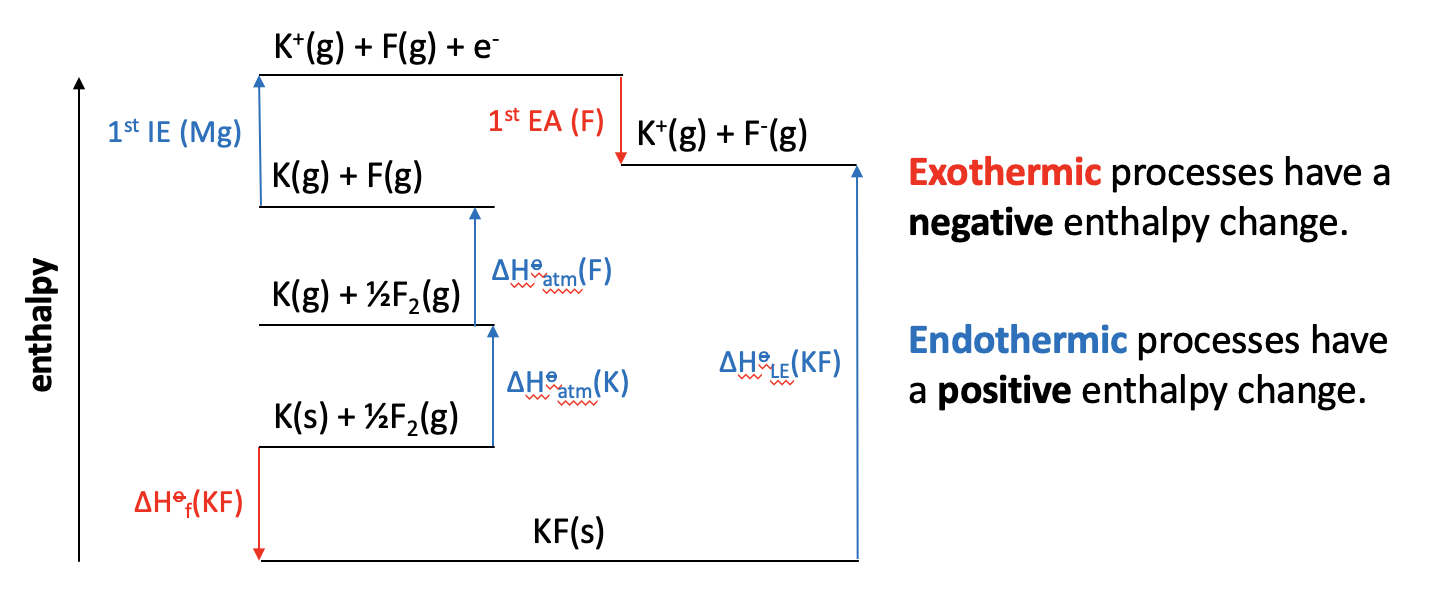
Which of the changes given is represented by the letter Y on the Born Haber cycle diagram below:
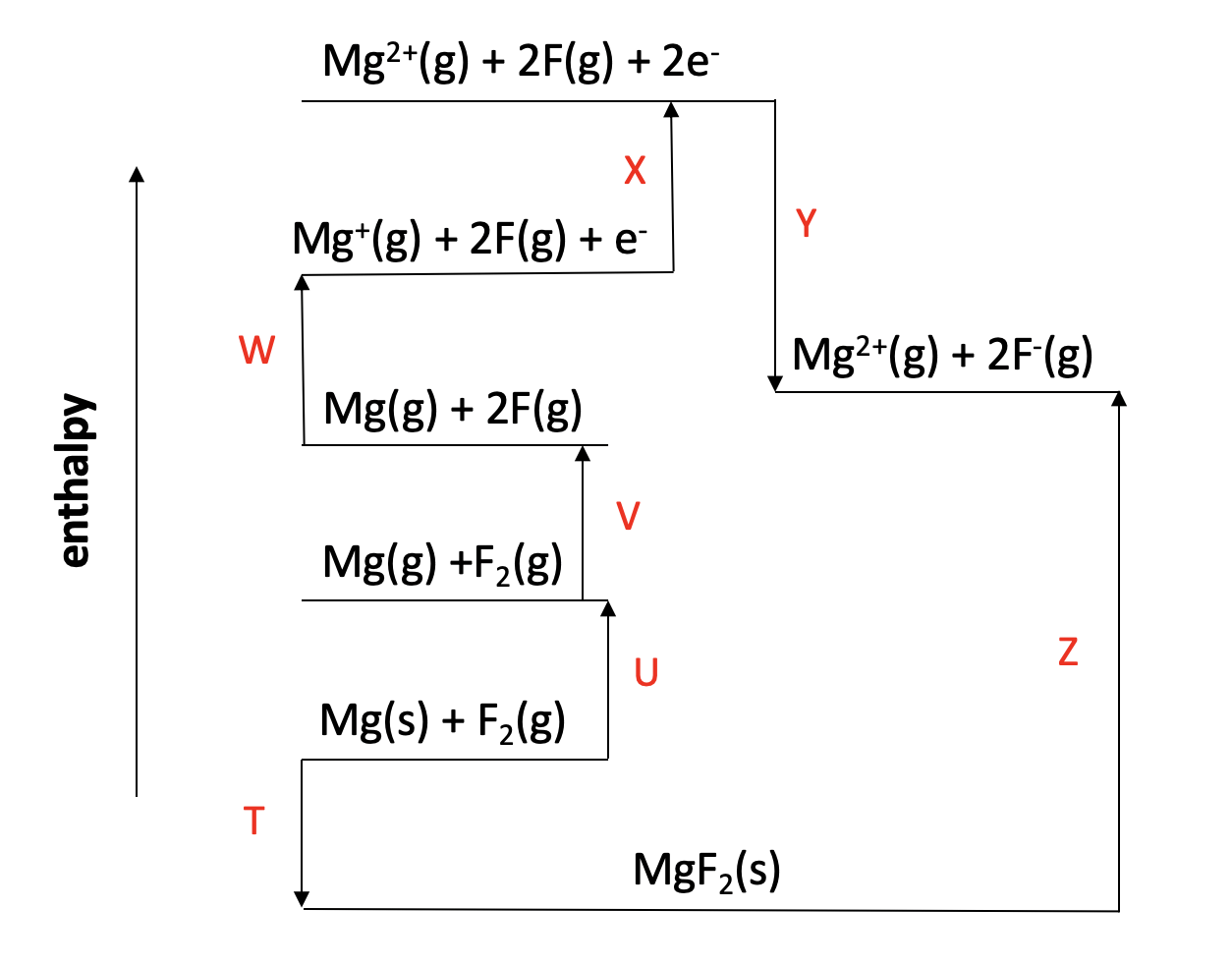
The enthalpy change Y on the diagram shows the reaction Mg2+(g) + 2F(g) + 2e− → Mg2+(g) + 2F−(g). The magnesium ion does not change. The electrons are gained by the fluorine atoms. so this is twice the 1st electron affinity for fluorine (F(g) + e− → F−(g)).
The correct answer is therefore 1st electron affinity for fluorine × 2.
The enthalpy change definitions all need to be learned.
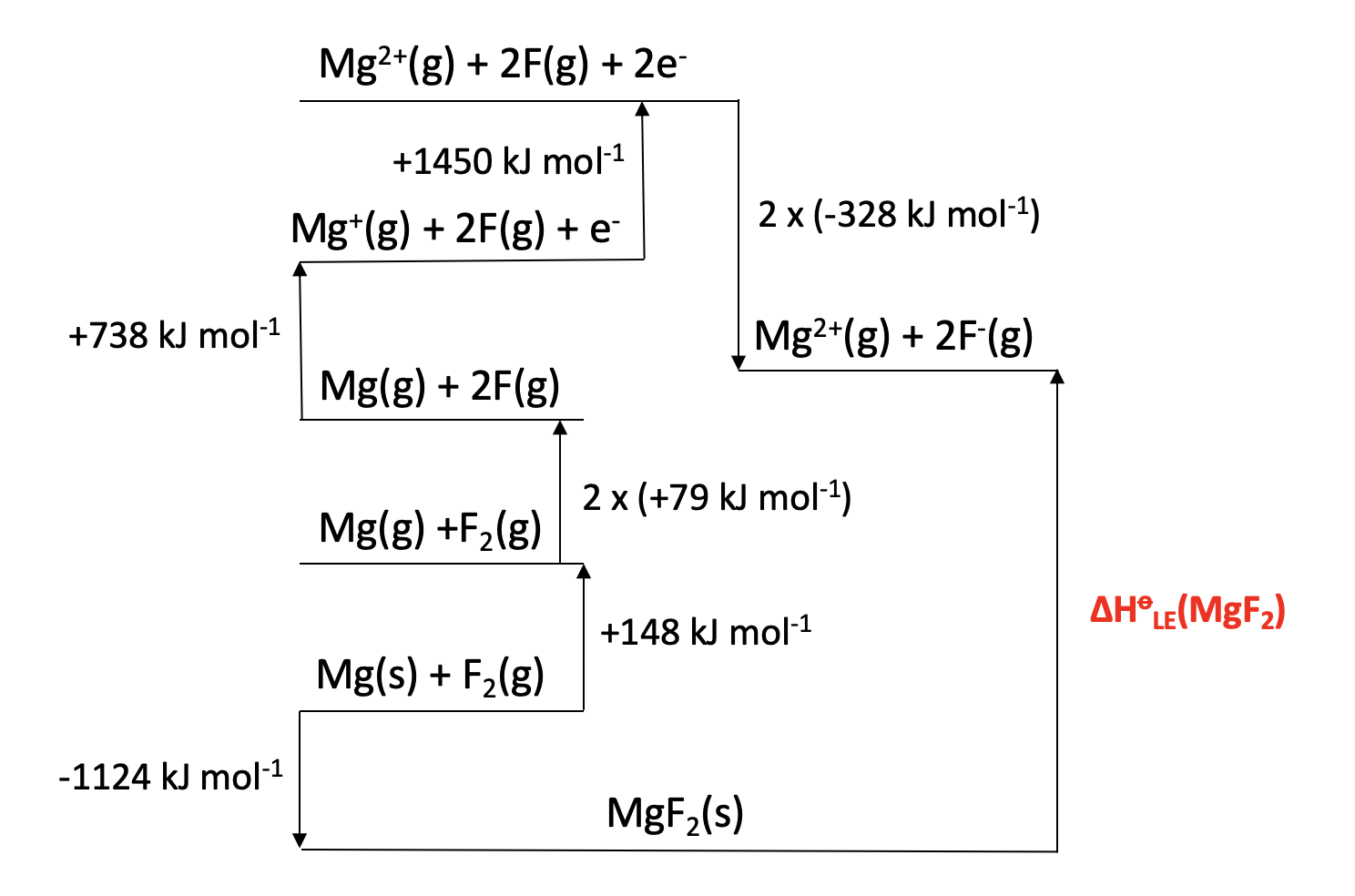
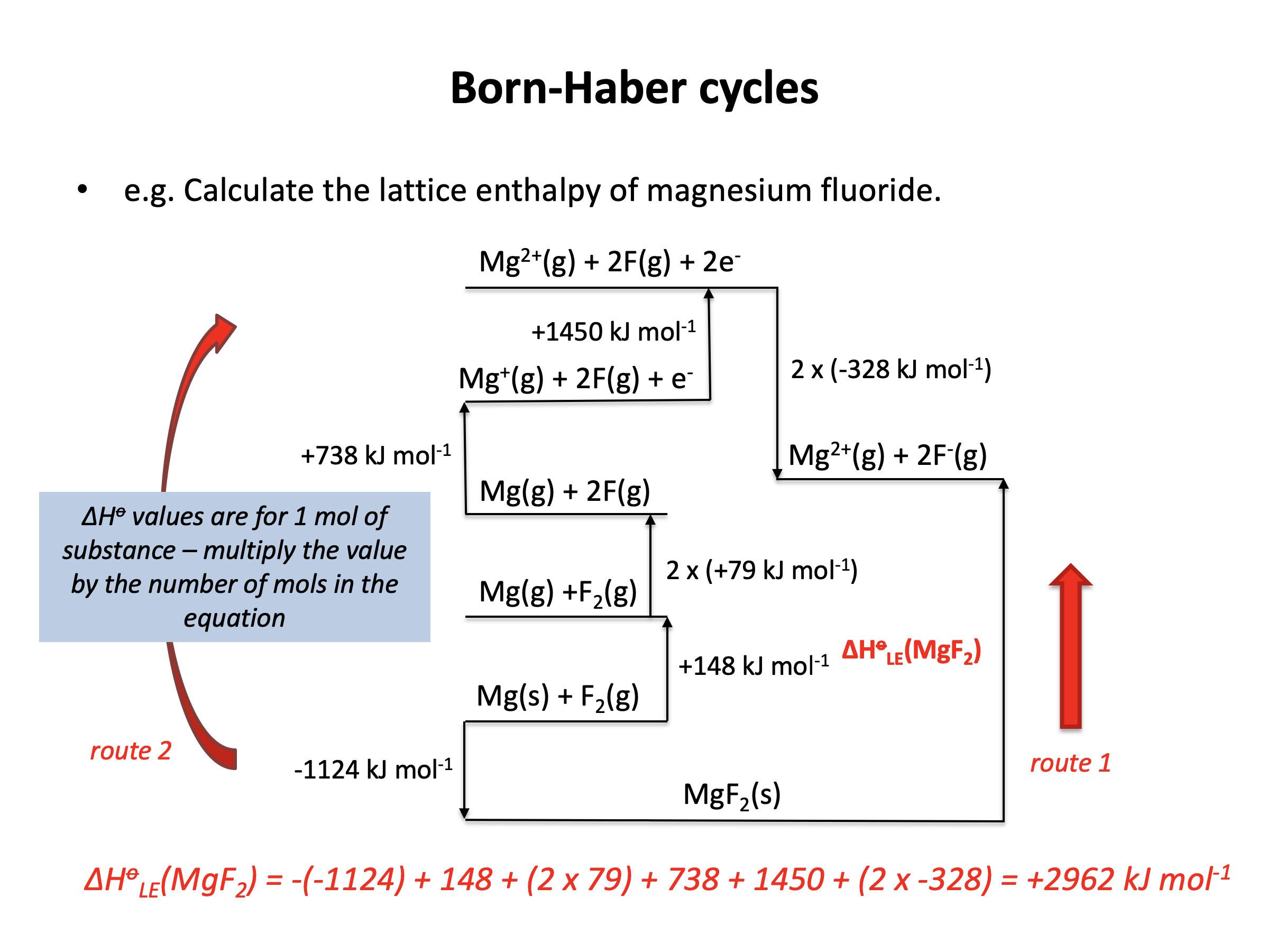
Calculator question: Given the enthaply changes below, what is the lattice enthalpy (in kJ mol−1) for magnesium fluoride, MgF2?
| Enthalpy change | Value (kJ mol−1) | |
| Enthalpy of formation (magnesium fluoride) | −1124 | |
| Enthalpy of atomisation (magnesium) | +148 | |
| Enthalpy of atomisation (fluorine) | +79 | |
| 1st ionisation enthalpy (magnesium) | +738 | |
| 2nd ionisation enthalpy (magnesium) | +1450 | |
| 1st electron affinity (fluorine) | −328 |
ΔHlatt(MgF2) = −(−1124) + 148 + (2×79) + 738 + 1450 + (2×−328) = +2962 kJmol−1
+2962 is therefore the correct answer.
Incorrect answers
+3290 is obtained if −328 is used instead of 2× −328 (not using twice the electron affinity for fluorine).
+2883 is obtained if +79 is used instead of 2× +79 (not using twice the atomisation energy for fluorine).
+3211 is obtained if −328 is used instead of 2× −328 and +79 is used instead of 2× +79 (not using twice the electron affinity and twice the atomisation energy for fluorine).
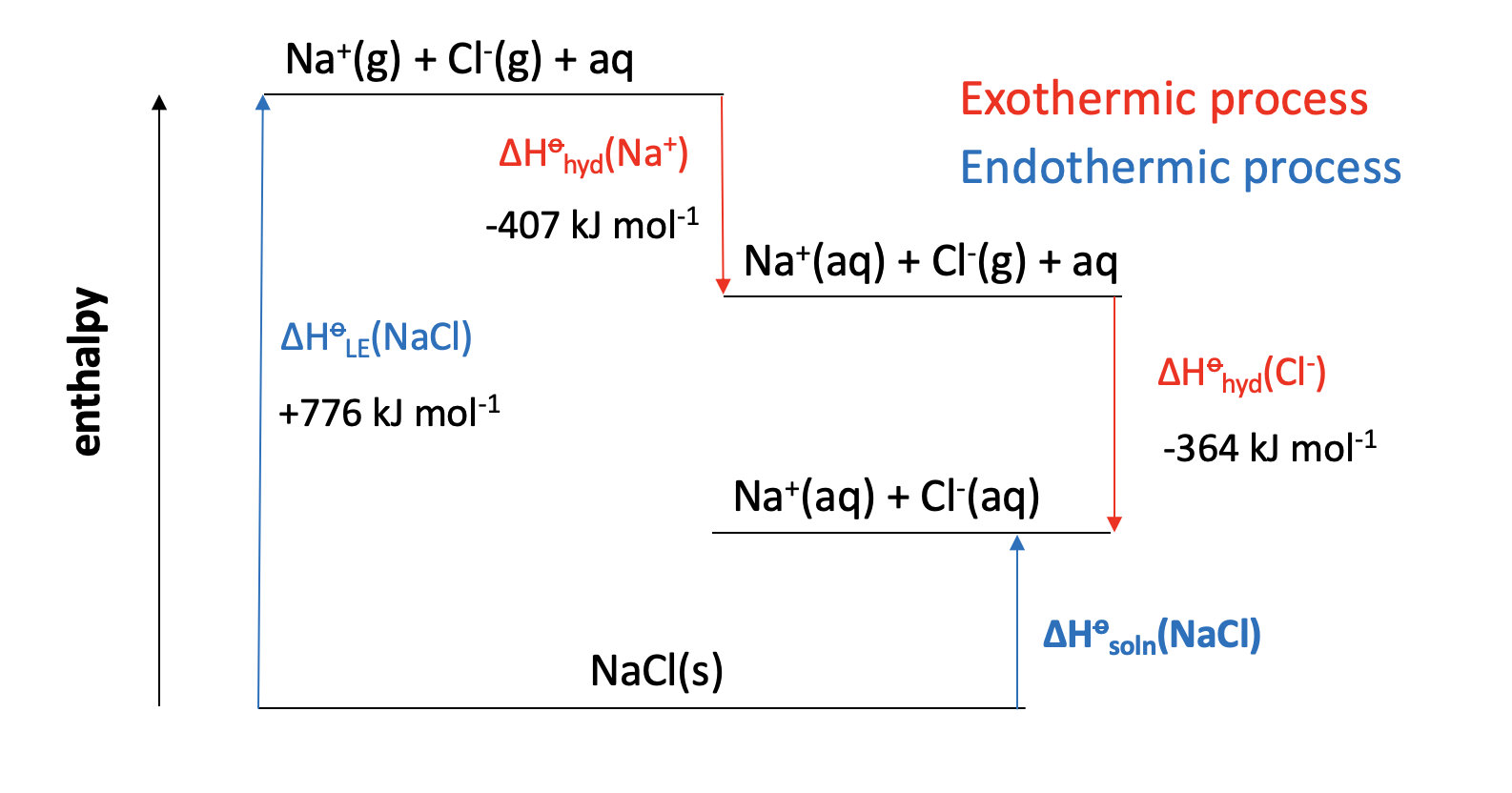
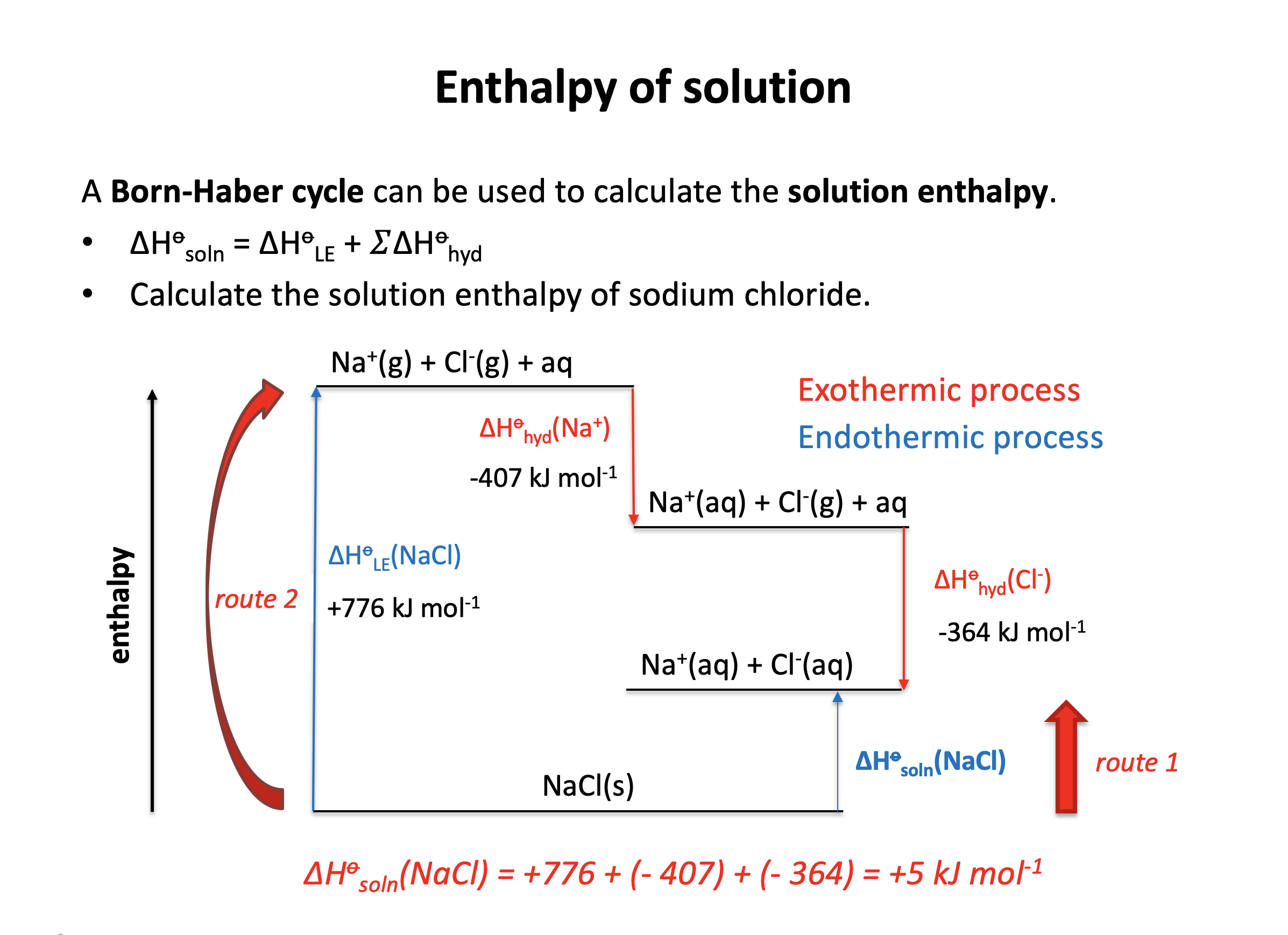
Calculator question: Given the enthalpy changes below, what is the enthalpy of solution in water (in kJ mol−1) for sodium chloride, NaCl?
| Enthalpy change | Value (kJ mol−1) | |
| Lattice enthalpy (sodium chloride) | +776 | |
| Enthalpy of hydration (sodium ion) | −407 | |
| Enthalpy of hydration (chloride ion) | −364 |
ΔHsoln(NaCl) = +776 + (−407) + (−364) = +5 kJmol−1
+5 is therefore the correct answer.
Incorrect answers
−5 is obtained if signs are inverted.
−1547 is obtained if the sign for the lattice enthalpy is inverted (using −776 instead of +776).
+1547 is obtained if the sign for both hydration enthalpies are inverted (using + instead of −)
Which of the equations below represents the standard enthalpy change of hydration for sodium chloride?
Standard enthalpy change of hydration is a definition that needs to be learned:
Standard enthalpy of hydration is the enthalpy change when one mole of aqueous solution is formed from ions in the gaseous state.
Thus Na+(g) + Cl−(g) → NaCl(aq) is the correct answer.
Standard enthalpy change of solution is also definition that needs to be learned (and not confused with hydration):
Standard enthalpy of solution is the enthalpy change when one mole of aqueous solution is formed from one mole of solute in its standard state. (e.g. NaCl(s) → NaCl(aq) for sodium chloride)
Which of the equations below represents the standard enthalpy of atomisation of bromine?
Standard enthalpy of atomisation is a definition that needs to be learned:
Standard enthalpy of atomisation is the enthalpy change when one mole of gaseous atoms is formed from the element in its standard state (at 100kPa and 298K).
Thus ½Br2(l) → Br(g) is the correct answer.
Incorrect answers:
Br2(l) → 2Br(g) shows two moles of bromine atoms (rather than one mole).
½Br2(g) → Br(g) shows bromine molecules in the gaseous state (rather than liquid; its standard state).
Br2(g) → 2Br(g) shows bromine molecules in the gaseous state (rather than liquid; its standard state) and shows two moles of bromine atoms (rather than one mole).
Which of the equations below represents the second electron affinity of oxygen?
First electron affinity and second electron affinity are definitions that need to be learned:
First electron affinity is the enthalpy change when one electron is gained by each atom in one mole of gaseous atoms.
Second electron affinity is the enthalpy change when one electron is gained by each 1− ion in one mole of gaseous ions.
Thus O−(g) + e− → O2−(g) is the correct answer.
Paper 1
Core (SL&HL): Energetics core (SL and HL) paper 1 questions
AHL (HL only): Energetics AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Energetics core (SL & HL) paper 2 questions
AHL (HL only): Energetics AHL (HL only) paper 2 questions
How much of Energy cycles have you understood?













 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn