 A chemical reaction involves the breaking of bonds in reactants and the forming of bonds in products. If the energy required to break bonds in reactants is less than the energy released when bonds are formed in products, the reaction is exothermic. Conversely, if the energy required to break bonds in reactants is more than the energy released when bonds are formed in products, the reaction is endothermic.
A chemical reaction involves the breaking of bonds in reactants and the forming of bonds in products. If the energy required to break bonds in reactants is less than the energy released when bonds are formed in products, the reaction is exothermic. Conversely, if the energy required to break bonds in reactants is more than the energy released when bonds are formed in products, the reaction is endothermic.
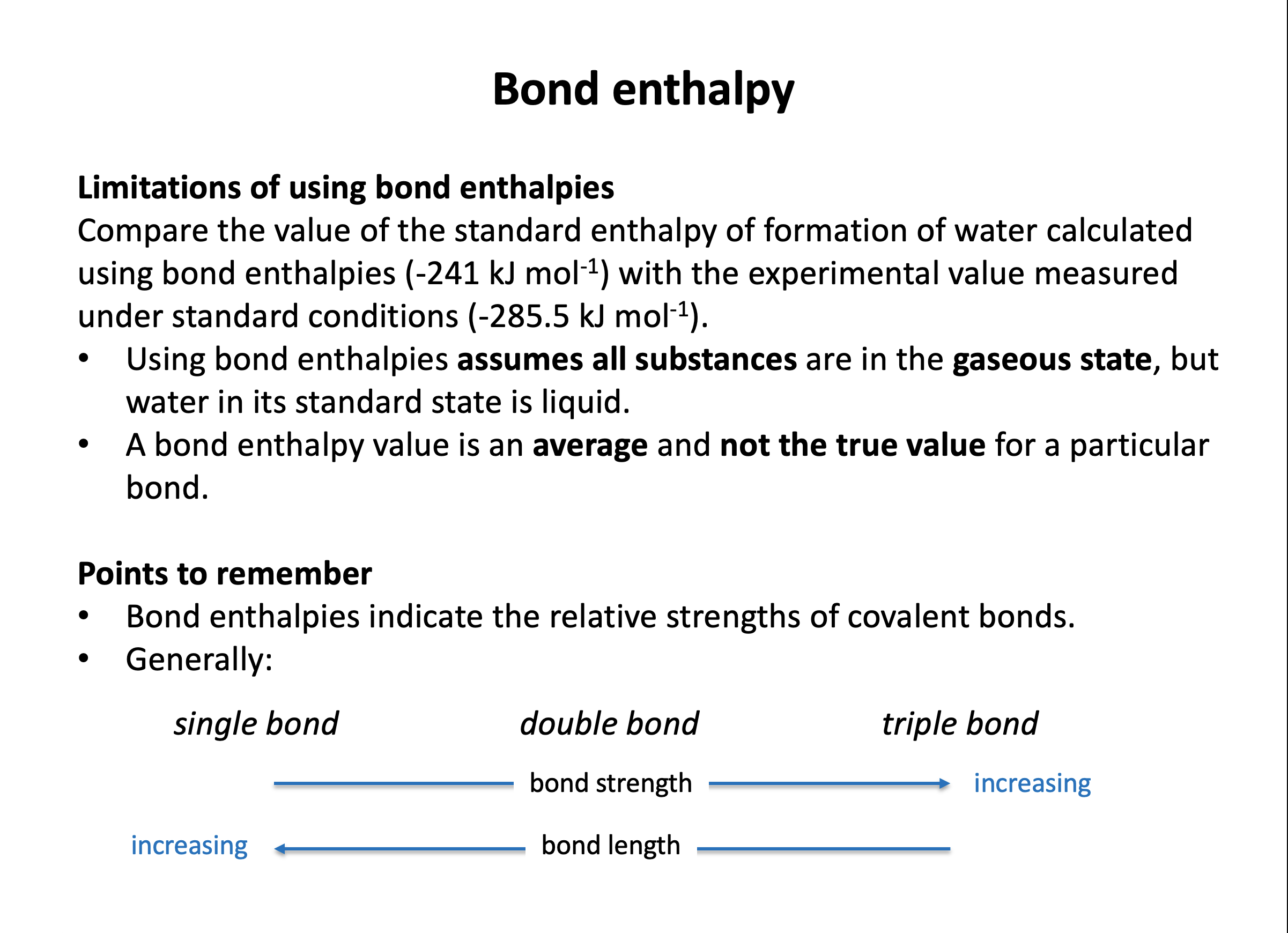
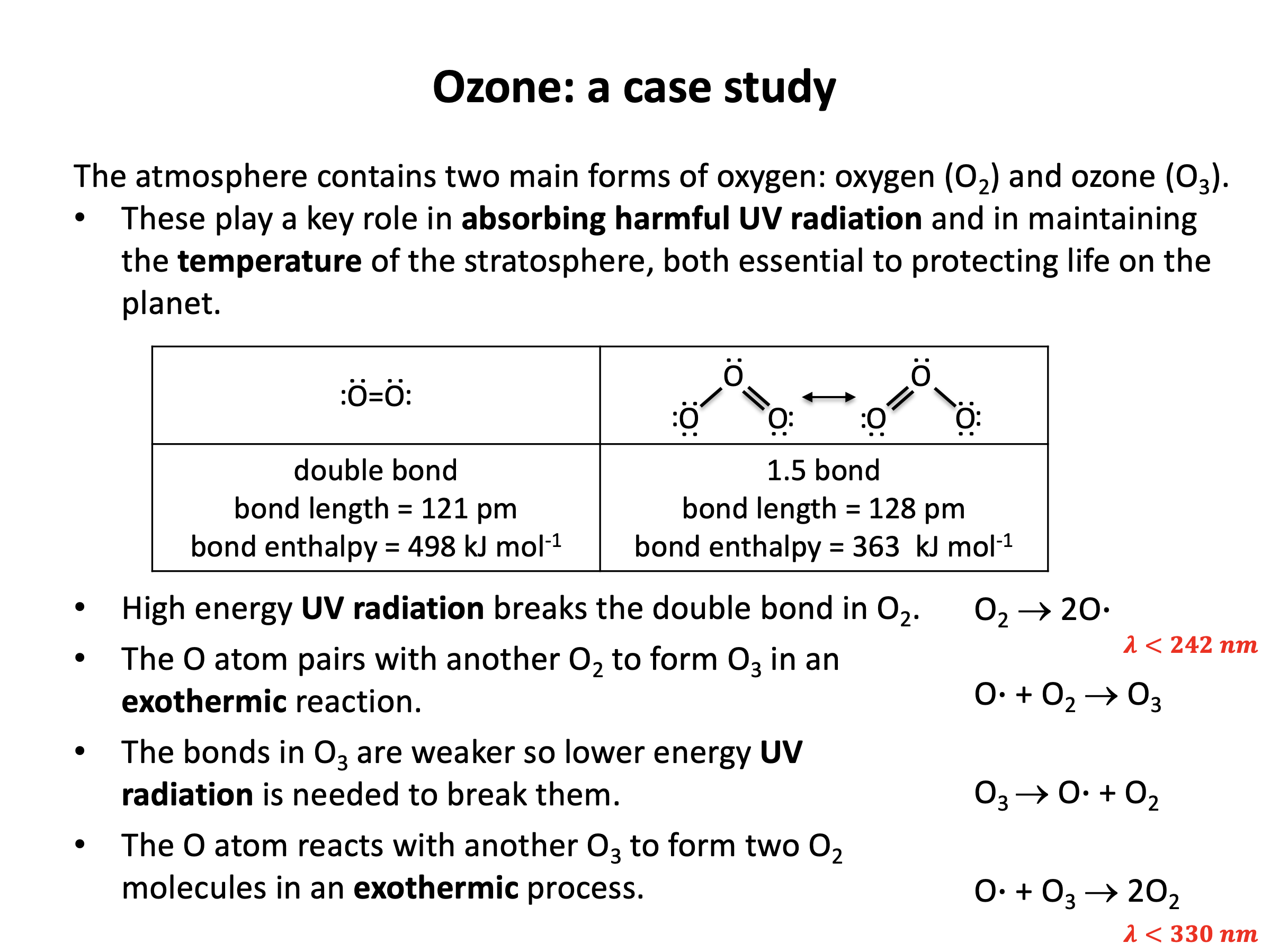
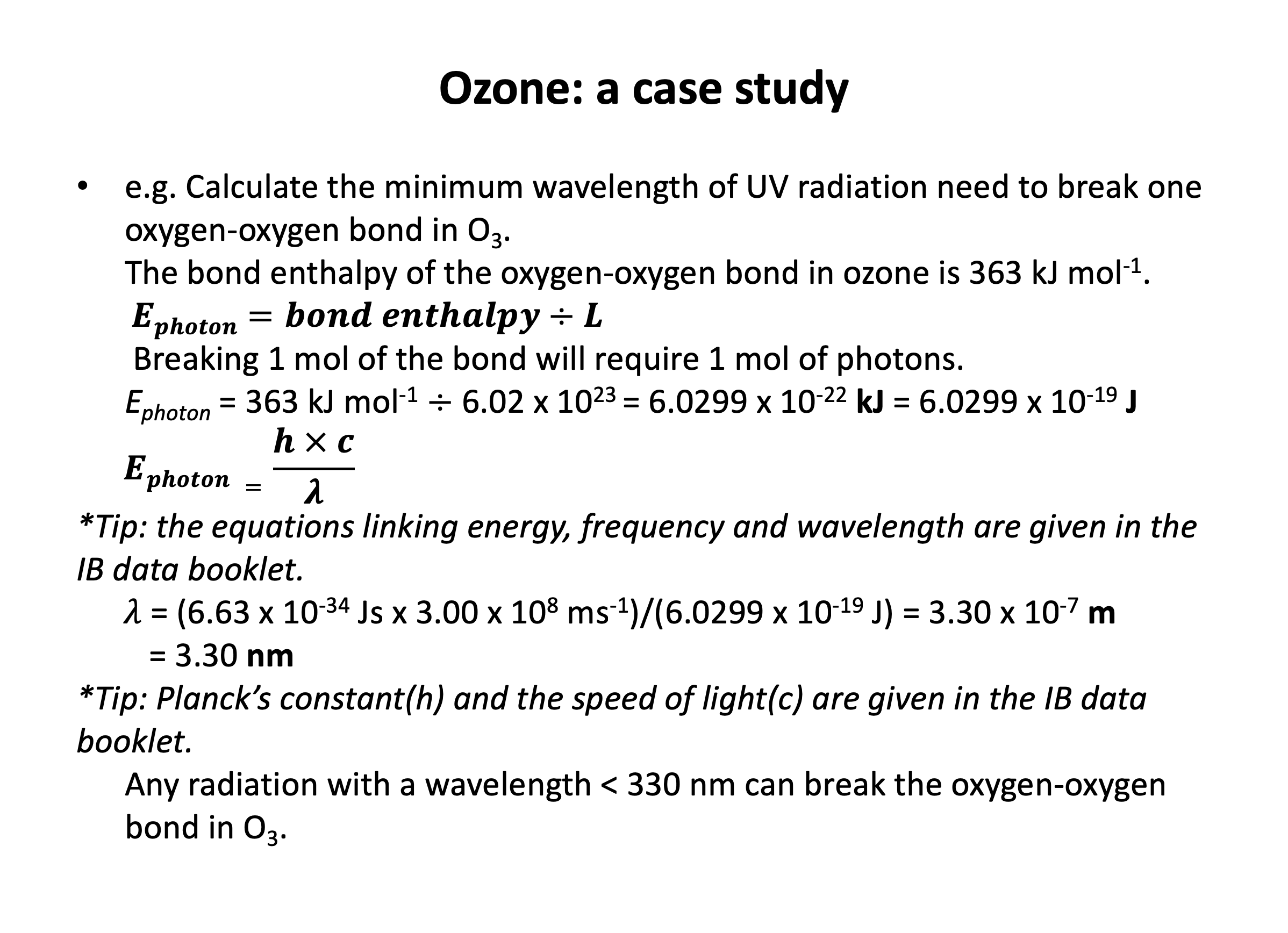
Bond enthalpy calculations are straight forward, but it is easy to select the wrong value (for example C-C when you need C=C) if you do not draw out the chemical structures in full. Refer to revision card 3 to help you organise your working. You should understand the importance of ozone and molecular oxygen in the atmosphere and be able to calculate the wavelengths of UV radiation needed to break the oxygen-oxygen bonds in each. Using revision card 6 as a model, practise writing out the calculation repeatedly, explaining it as you go.
Ensure you are confident using the terms below and learn the asterisked* definitions
average bond enthalpy*
Which of the following are correctly included in the definition of bond enthalpy?
1: Bond enthalpy is an average
2: Bond enthalpy is calculated for one bond
3: Bond enthalpy is given in the gaseous state
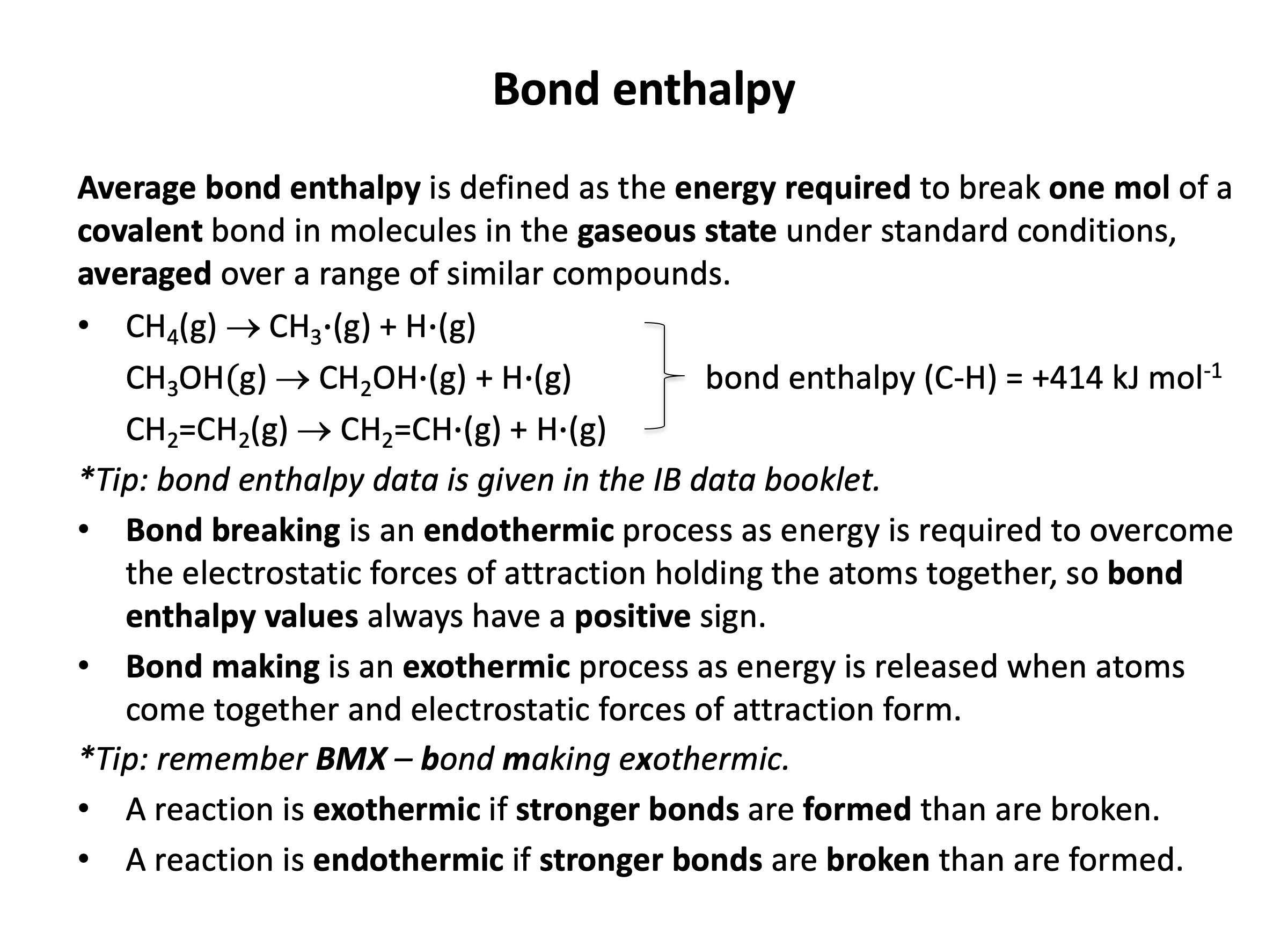
Bond enthalpy is defined as the average enthalpy required to break one mole of covalent bonds in the gaseous state.
Thus the correct answer is 1 and 3 only. Statement 2 suggests 'one bond' rather than one mole of bonds.
Which of the following statements is correct?
Breaking bonds requires overcoming attractive forces so bond breaking is endothermic and has a positive ΔH (requires energy input into the system). Making bonds generates attractive forces which releases energy from the system into the surroundings so is exothermic and has a negative ΔH.
Thus 'Bond breaking is endothermic and bond making is exothermic' is the correct answer.
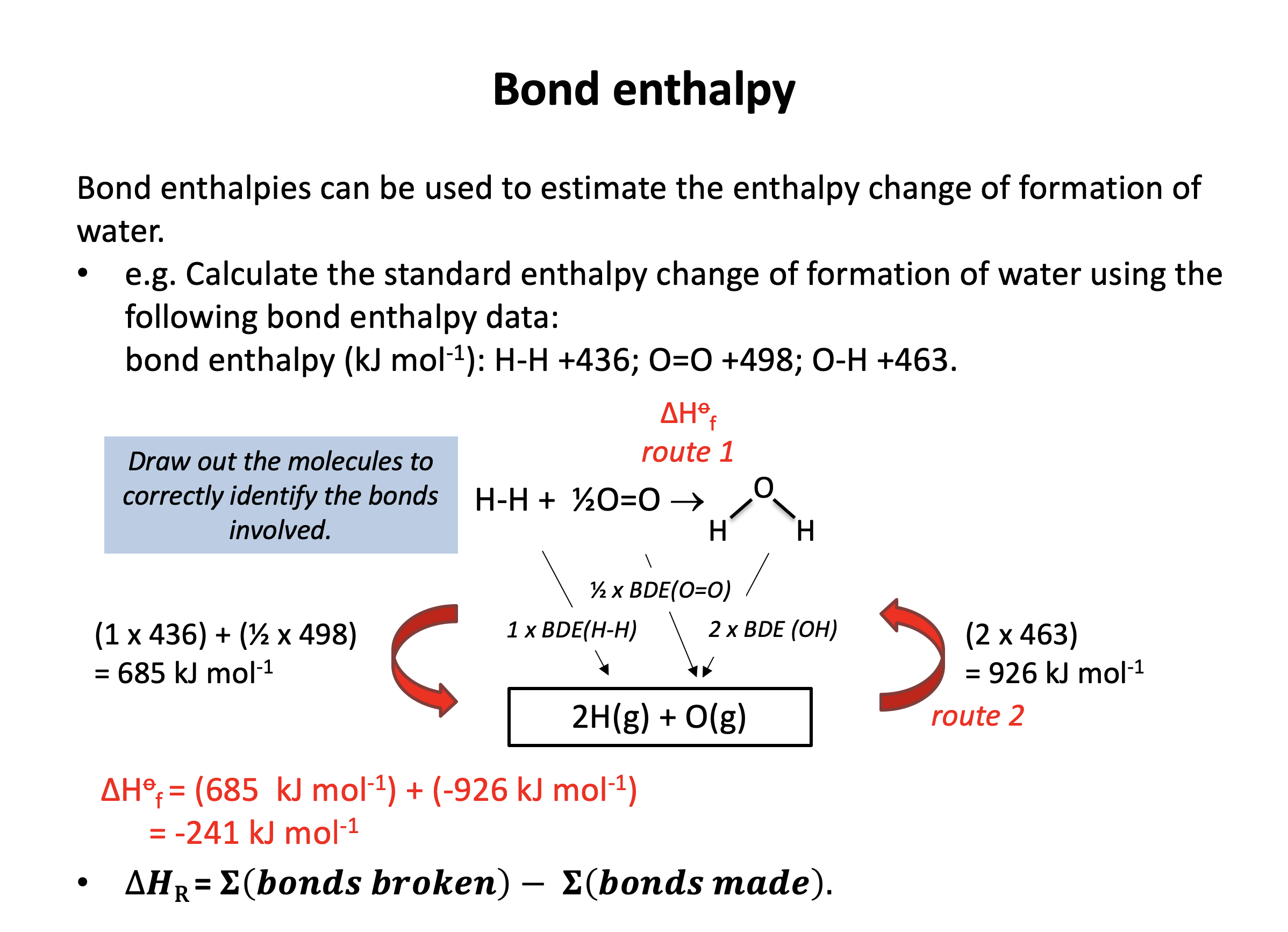
Estimate the enthalpy change (in kJ mol−1) for the following reaction using average bond enthalpies (given in the data book, and below).
2H2(g) + O2(g) → 2H2O(g)
Bond enthalpies in kJ mol−1 that you may require: H−H 436 / O=O 498 / O−O 144 / O−H 463
Total energy change = bonds broken − bonds made
Bonds present in reactants: 2×H−H, 1×O=O
Bonds present in products: 4×O−H
(notice that there are no O−O bonds present in reactants or products)
Assuming all the bonds in reactants are broken and all bonds in products are made:
Magnitude of enthalpy for bonds broken: 2×436 + 1×498 = 1370
Magnitude of enthalpy for bonds made: 4×463 = 1852
Estimate of enthalpy change = 1370 − 1852 = −482 kJ mol−1
Thus the correct answer is −482
Incorrect answers:
−241 is half the value (enthalpy for one mole of water being formed).
−836 is the value obtained when 144 (O−O) is used instead of 498 (O=O).
+444 is the value obtained when 2×O−H, 926, is used instead of 4×O−H, 1852.
Which of the following is likely to best represent the average bond enthalpy for the C−H bond in methane?
Average bond enthalpy is defined as the average enthalpy required to break one mole of covalent bonds in the gaseous state.
So the best representation is going to be a chemical change showing the average breaking of one mole of C−H bonds in methane the gaseous state.
CH4(g) → CH3(g) + H(g) shows a single C−H bond breaking and by definition (stoichiometric coefficients) we have one mole of methane (CH4) and thus one mole of C−H bonds breaking. But, when we break the bonds in any molecule one-by-one the bonds will all have different enthalpies as the environment of the bonds is changing.
Therefore we need to break all four bonds in methane and find the average enthalpy:
¼CH4(g) → ¼C(g) + H(g) is thus the correct answer.
Incorrect answers
CH4(g) → C(g) + 4H(g) and CH4(g) → C(g) + 2H2(g) both show four moles of C−H bonds breaking, with the hydrogen products shown as atoms or molecules.
Estimate the enthalpy change (in kJ mol−1) for the reaction below using average bond enthalpies (given in the data book, and below).
H2(g) + O2(g) → H2O2(g)
Bond enthalpies in kJ mol−1 that you may require: H−H 436 / O=O 498 / O−O 144 / O−H 463
Total energy change = bonds broken − bonds made
Bonds present in reactants: 1×H−H, 1×O=O
Bonds present in products: 2×O−H, 1×O−O
Assuming all the bonds in reactants are broken and all bonds in products are made:
Magnitude of enthalpy for bonds broken: 1×436 + 1×498 = 934
Magnitude of enthalpy for bonds made: 2×463 + 1×144 = 1070
Estimate of enthalpy change = 934 − 1070 = −136 kJ mol−1
Thus the correct answer is −136
Incorrect answers:
−490 is the value obtained when 498 (O=O) is used instead of 144 (O−O) in the products (bonds made) or −490 is also the value obtained when 144 (O−O) is used instead of 498 (O=O) in the reactants (bonds broken).
−844 is the value obtained when 498 (O=O) is used instead of 144 (O−O) in the products (bonds made) and 144 (O−O) is used instead of 498 (O=O) in the reactants (bonds broken).
+8 is the value obtained when 1×O−O (144) is missing from products (bonds made), that is only 2×O−H, 926, is used.
Which of the following statements best describes the action of oxygen (O2) and ozone (O3) in the atmosphere with respect to absorption of ultraviolet radiation from the sun?
Both oxygen and ozone absorb u/v radiation is the correct answer.
The double bond in oxygen is stronger than the '1½' bonds in ozone, but both oxygen and ozone will absorb ultraviolet radiation from the sun and the covalent bonds may break to form oxygen free radicals.
This is an important mechanism for the protection of life on the planet from the damaging (ionising) effects of ultraviolet light.
Which of the following statements best describes the relationship between oxygen (O2) and ozone (O3) in the atmosphere with respect to absorption of ultraviolet radiation from the sun?
Both oxygen and ozone will absorb ultraviolet radiation from the sun and the covalent bonds may break to form oxygen free radicals.
The double bond in oxygen is stronger than the '1½' bonds in ozone. So it takes ultraviolet radiation of higher energy to break the bonds in oxygen than in ozone. Energy of light is inversely proportional to wavelength (higher energy = lower wavelength), so the correct answer is:
Ozone has weaker bonds than oxygen so absorbs u/v radiation of a higher wavelength.
Paper 1
Core (SL&HL): Energetics core (SL and HL) paper 1 questions
AHL (HL only): Energetics AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Energetics core (SL & HL) paper 2 questions
AHL (HL only): Energetics AHL (HL only) paper 2 questions
How much of Bond enthalpies have you understood?









 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn