 Reaction mechanisms are a fundamental part of organic chemistry. Each mechanism needs to be learned so that it can be re-written from scratch. This is quite a task and will take a lot of practice with blank sheets of paper. Use the revision cards and the videos to help understand what is happening. Ensure a thorough grasp of the core parts of the organic chemistry: Fundamentals of organic chemistry and Functional group chemistry before beginning revision here.
Reaction mechanisms are a fundamental part of organic chemistry. Each mechanism needs to be learned so that it can be re-written from scratch. This is quite a task and will take a lot of practice with blank sheets of paper. Use the revision cards and the videos to help understand what is happening. Ensure a thorough grasp of the core parts of the organic chemistry: Fundamentals of organic chemistry and Functional group chemistry before beginning revision here.
Ensure you are confident using the terms below and learn the asterisked* definitions
Curly double-headed arrow, Nucleophile*, Electrophile*, Carbocation, Asymmetric alkene, Positive inductive effect*, Transition state, Intermediate, Polar protic solvents, Polar aprotic solvents
Which of the following statements about organic mechanisms is/are correct?
1: A double-headed curly arrow represents the movement of a pair of electrons
2: A double-headed curly arrow can be used to show heterolytic fission
3: A double-headed curly arrow can be used to show homolytic fission
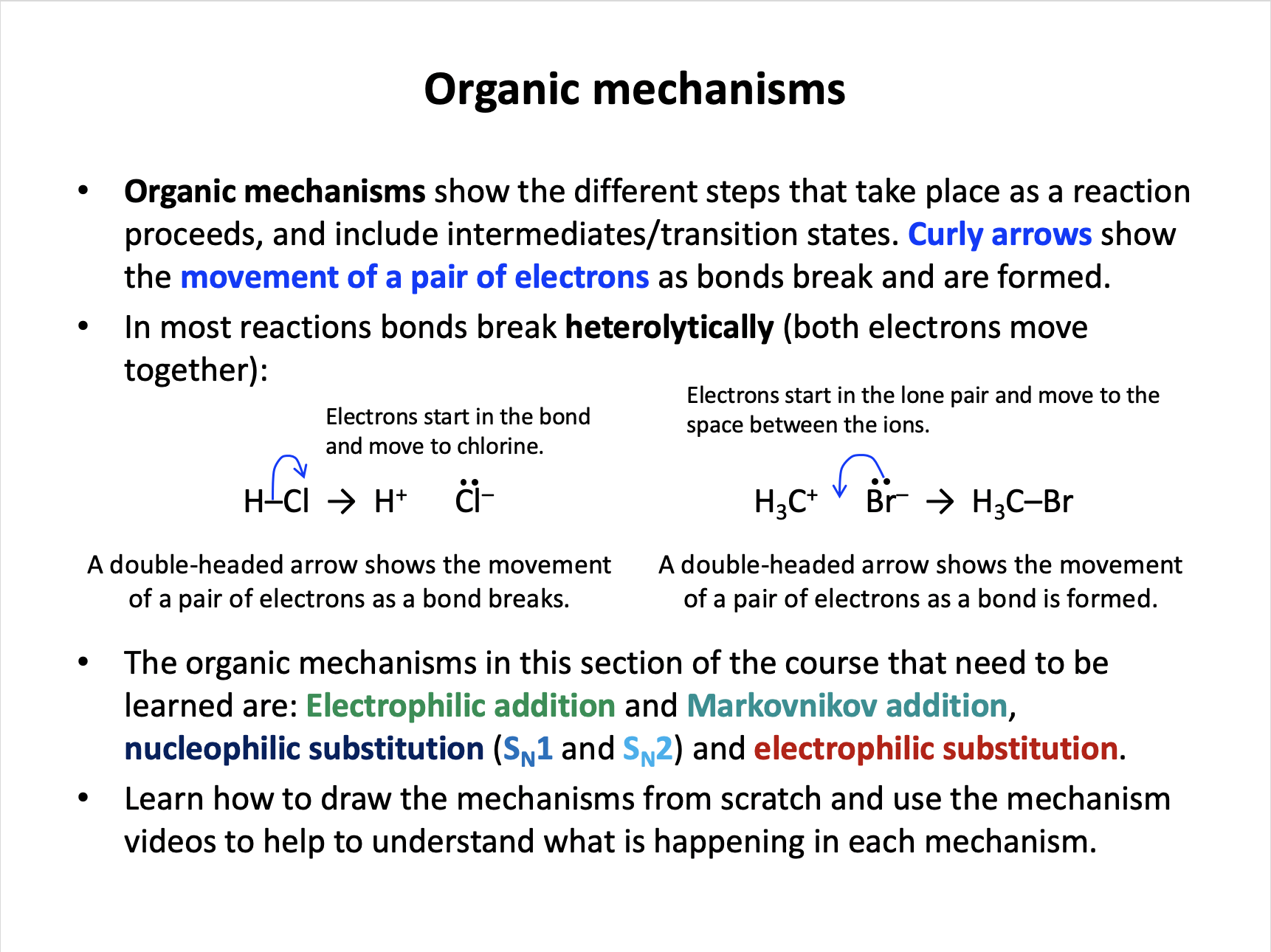
Organic mechanisms show the steps of a reaction. Curly arrows are used to show the movement of a pair of electrons as bonds break and are formed.
When a bond breaks heterolytically, both electrons move to one atom (one side of the bond) so a double-headed curly arrow may be used to show heterolytic fission.
When a bond breaks homolytically, one electron moves to each atom of the bond so a double-headed curly arrow may not be used to show heterolytic fission.
Therefore 1 and 2 only is the correct answer.
The diagram below represents a Markovnikov addition when H−Br reacts with propene, showing two possible routes for the mechanism. Which of the following statements about this mechanism is/are correct?
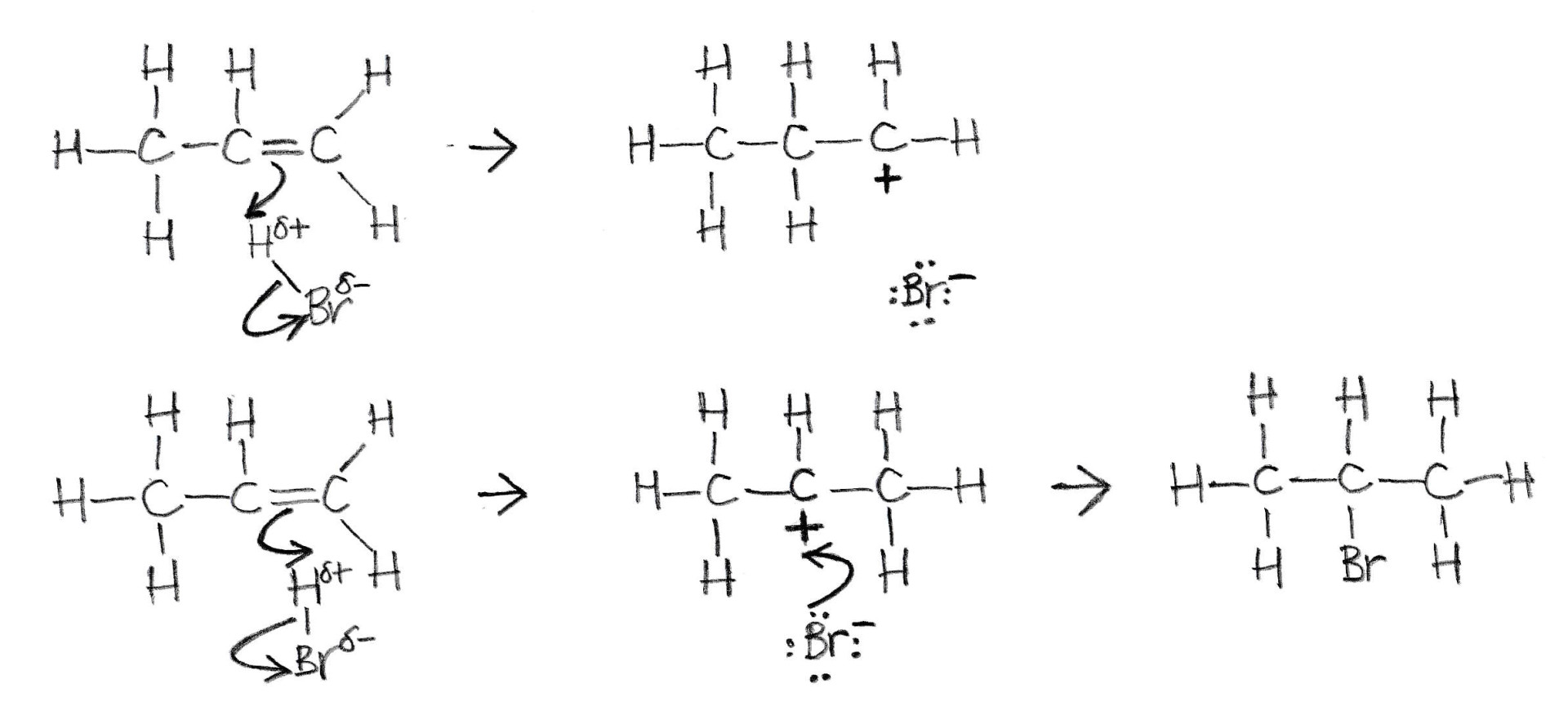
1: The preferred product in this reaction is 1-bromopropane
2: The secondary carbocation is more stable than the primary because of the positive inductive effect
3: The product for the unfinished first reaction mechanism would be 1-bromopropane
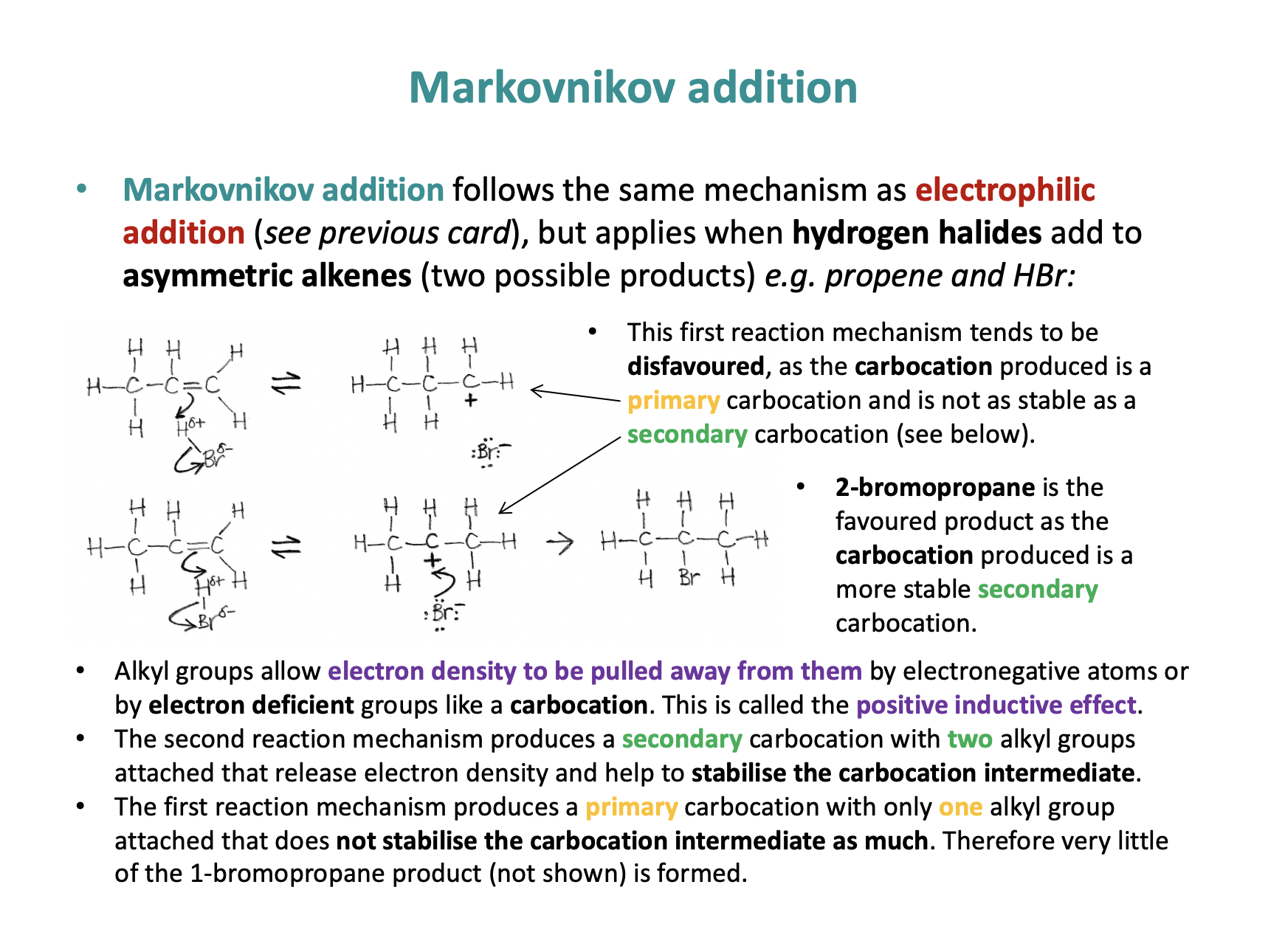
The mechanism shown is Markovnikov electrophilic addition.
Alkyl groups allow electron density to be pulled away from them by electron deficient atoms like a carbocation. This is called the positive inductive effect.
The second reaction mechanism produces a secondary carbocation with two alkyl groups attached that release electron density and help to stabilise the carbocation intermediate.
The first reaction mechanism produces a primary carbocation with only one alkyl group attached that does not stabilise the carbocation intermediate as much.
Therefore very little of the 1-bromopropane product (not shown) is formed.
2-bromopropane, shown in the second rmechanism is the preferred product.
Therefore 2 and 3 only is the correct answer.
Which of the following statements about this mechanism (below) is/are correct?
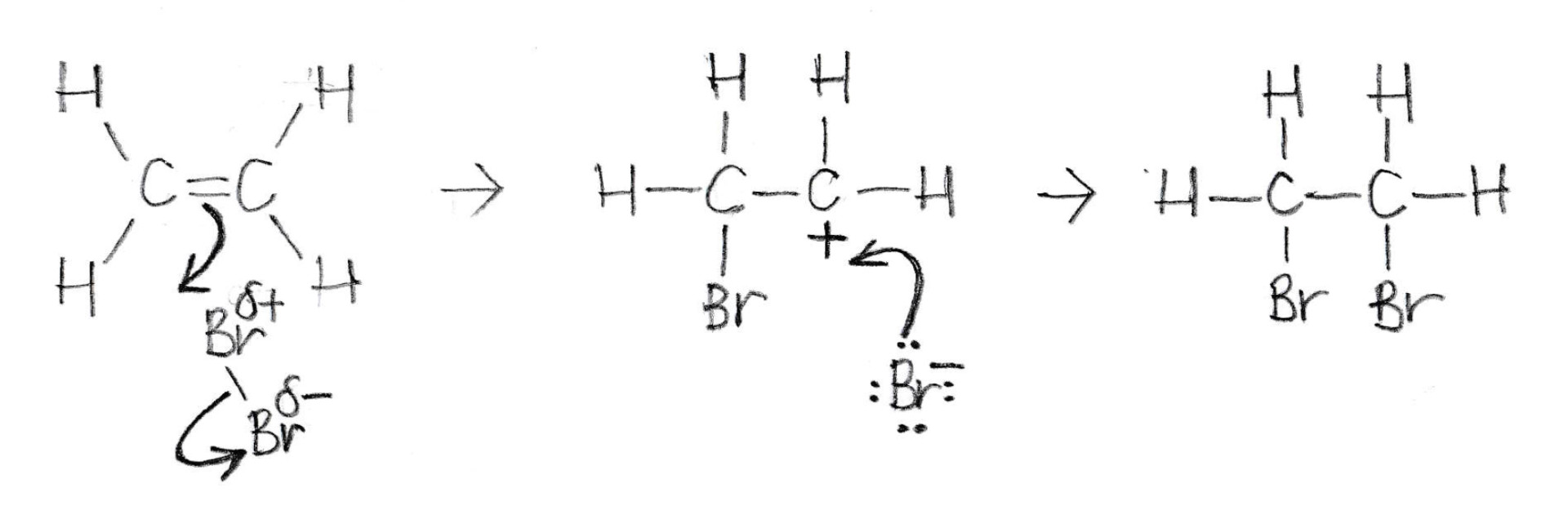
1: Brδ+ is an electrophile
2: Br− is a nucleophile
3: The bromine molecule is polarised because it approaches the electron-rich double bond.
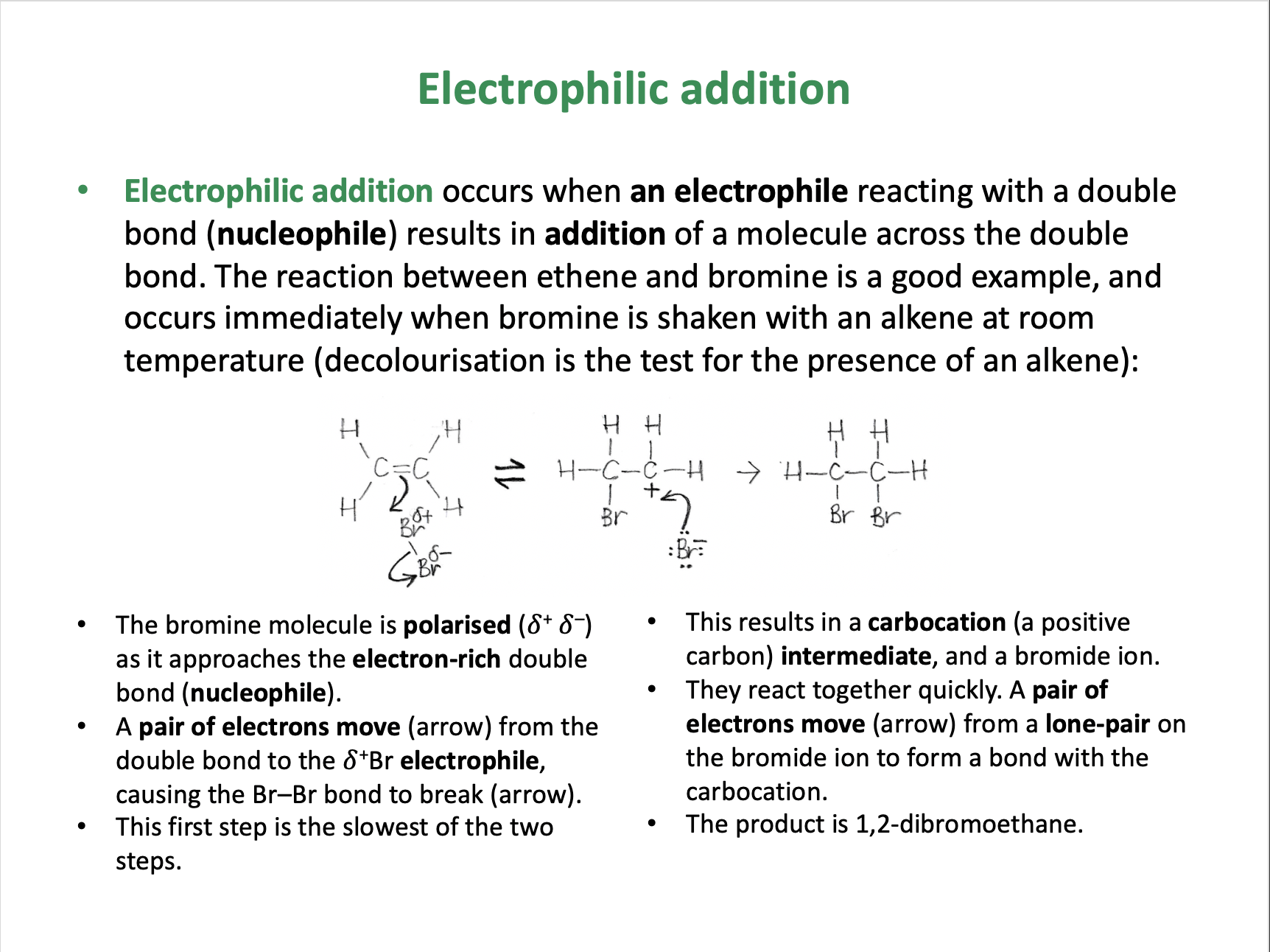
The mechanism shown is electrophilic addition.
The bromine molecule is polarised as it approaches the electron-rich double bond. A pair of electrons move (arrow) from the double bond to the δ+Br electrophile, causing the Br–Br bond to break (arrow). This first step is the slowest of the two steps.
This results in a carbocation (a positive carbon) intermediate, and a bromide ion.They react together quickly. A pair of electrons move (arrow) from a lone-pair on the bromide ion (nucleophile) to form a bond with the carbocation.The product is 1,2-dibromoethane.
Therefore 1, 2 and 3 is the correct answer.
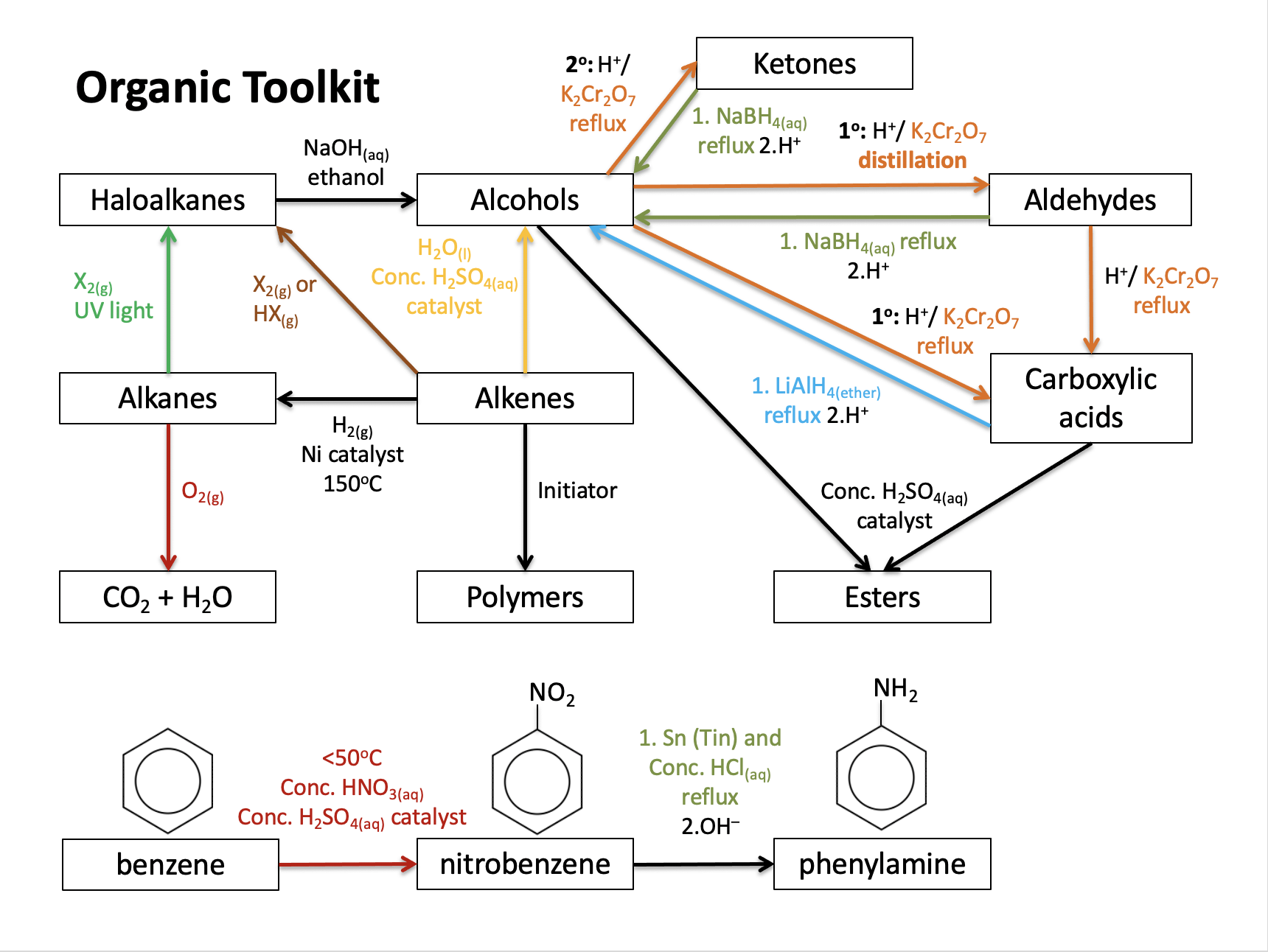
What is the correct order of reaction types (left to right) in the following series of reactions?
CH3CH2Cl → CH3CH2OH → CH3CHO
Reaction types and conditions need to be learned.
1-chloroethane to ethanol is a nucleophilic substitution reaction, and ethanol to ethanal is an oxidation reaction.
Therefore nucelophilic substitution; oxidation is the correct answer.
Which is likely to be the most preferred product in the reaction of but-1-ene with hydrogen bromide?
This is a Markovnikov electrophilic addition reaction mechanism.
The hydrogen bromide (H−Br) will add across the double bond. CH2BrCHBrCH2CH3 and CHBr2CH2CH2CH3 both contain two bromine atoms, so cannot be the product.
The mechanism will go preferentially via the secondary carbocation and produce 2-bromobutane (CH3CHBrCH2CH3) rather than 1-bromobutane (CH2BrCH2CH2CH3) which is formed via the primary carbocation.
Therefore CH3CHBrCH2CH3 is the correct answer.
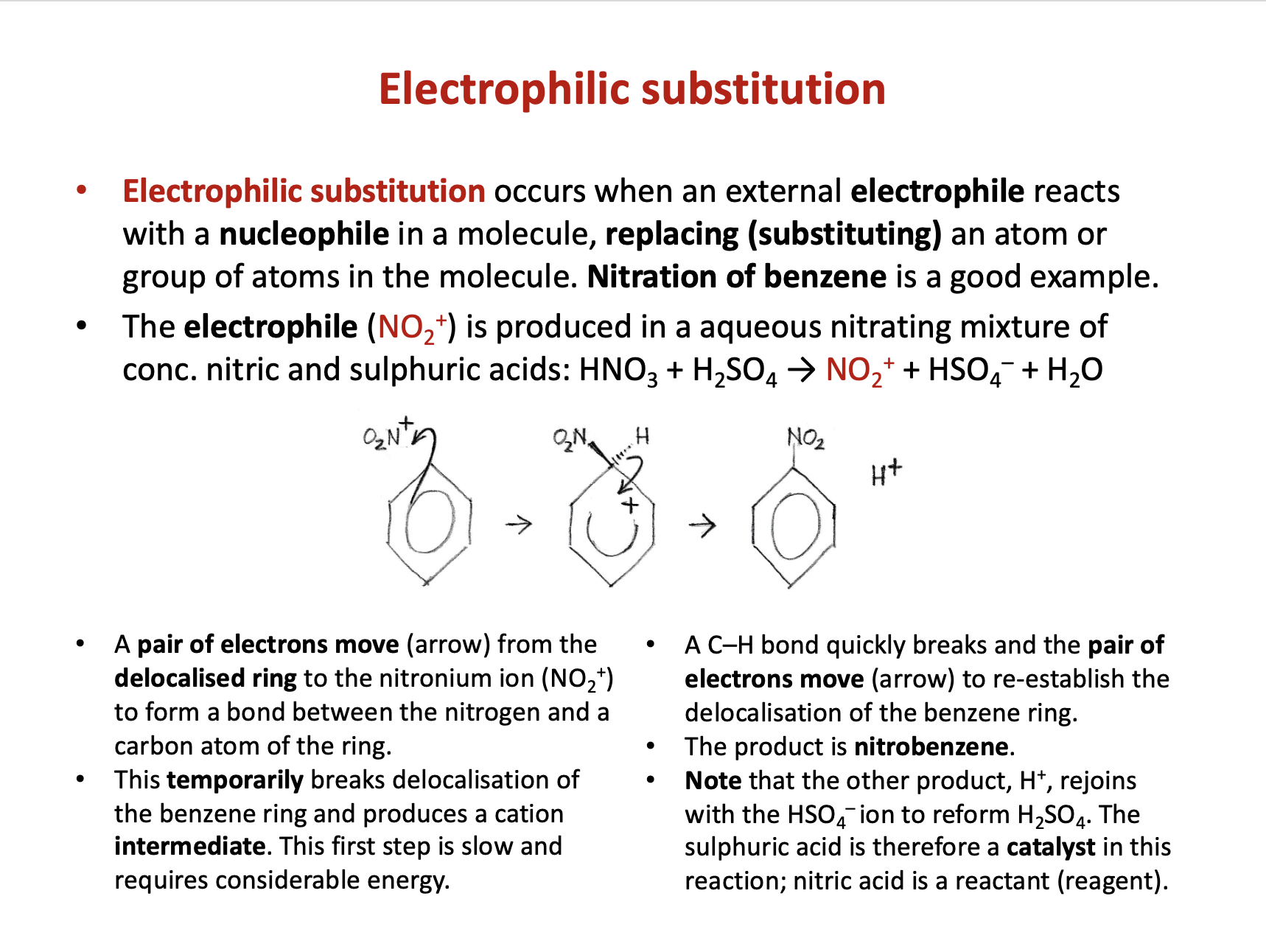
Which of the compounds below are more likely to undergo substitution rather than addition reactions?
1: CH2CH2
2: CH3CH2Cl
3: C6H6
Addition reactions only occur in molecules with double bonds. CH2CH2 is ethene, has a double bond and tends to undergo addition reactions.
CH3CH2Cl is chloroethane, a haloalkane. It does not have a double bond and tends to react via nucleophilic substitution reactions.
C6H6 is benzene. It has a delocalised ring and so does not tend to react via addition reactions, but rather by electrophilic substitution.
Therefore 2 and 3 only is the correct answer.
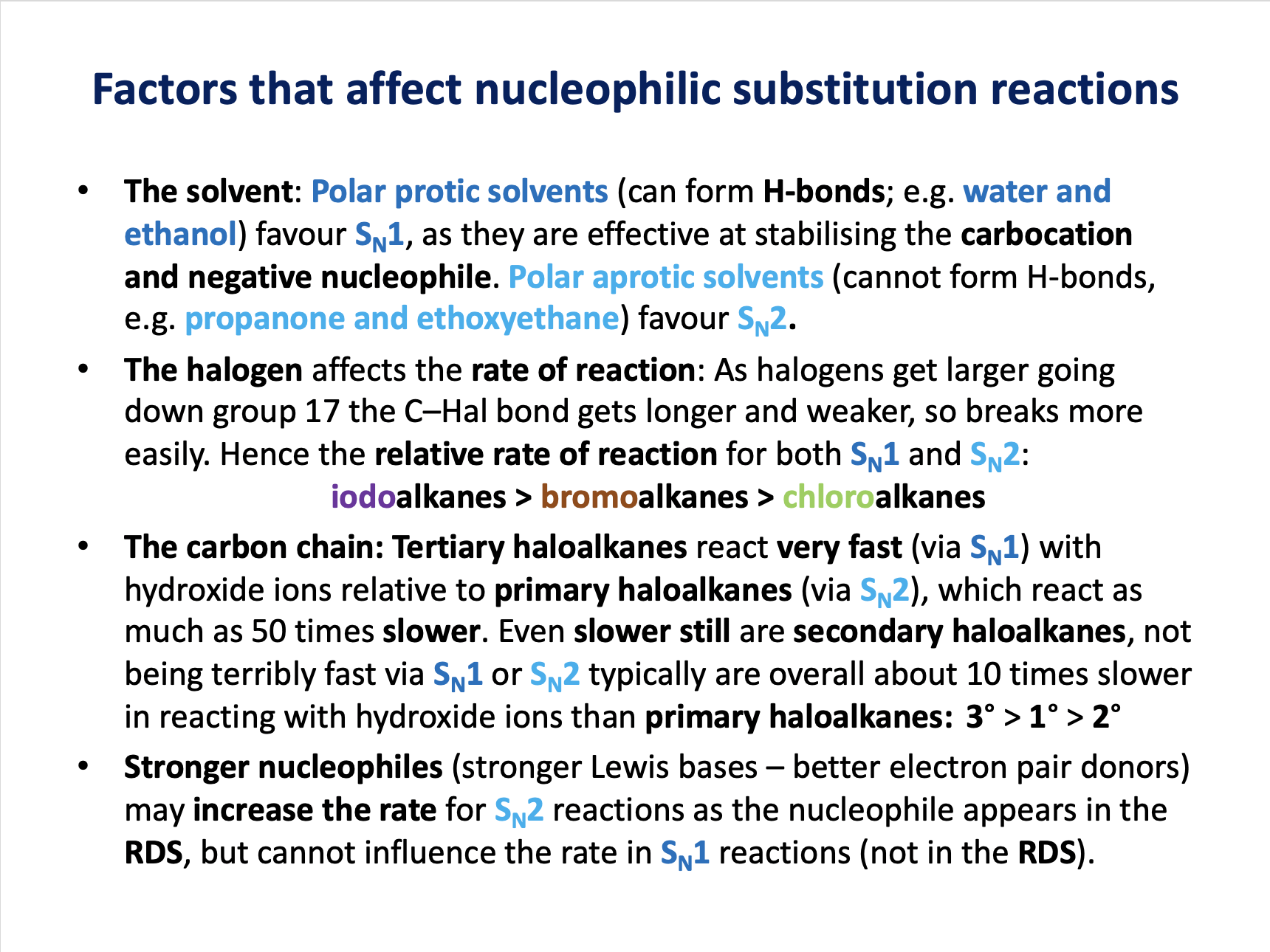
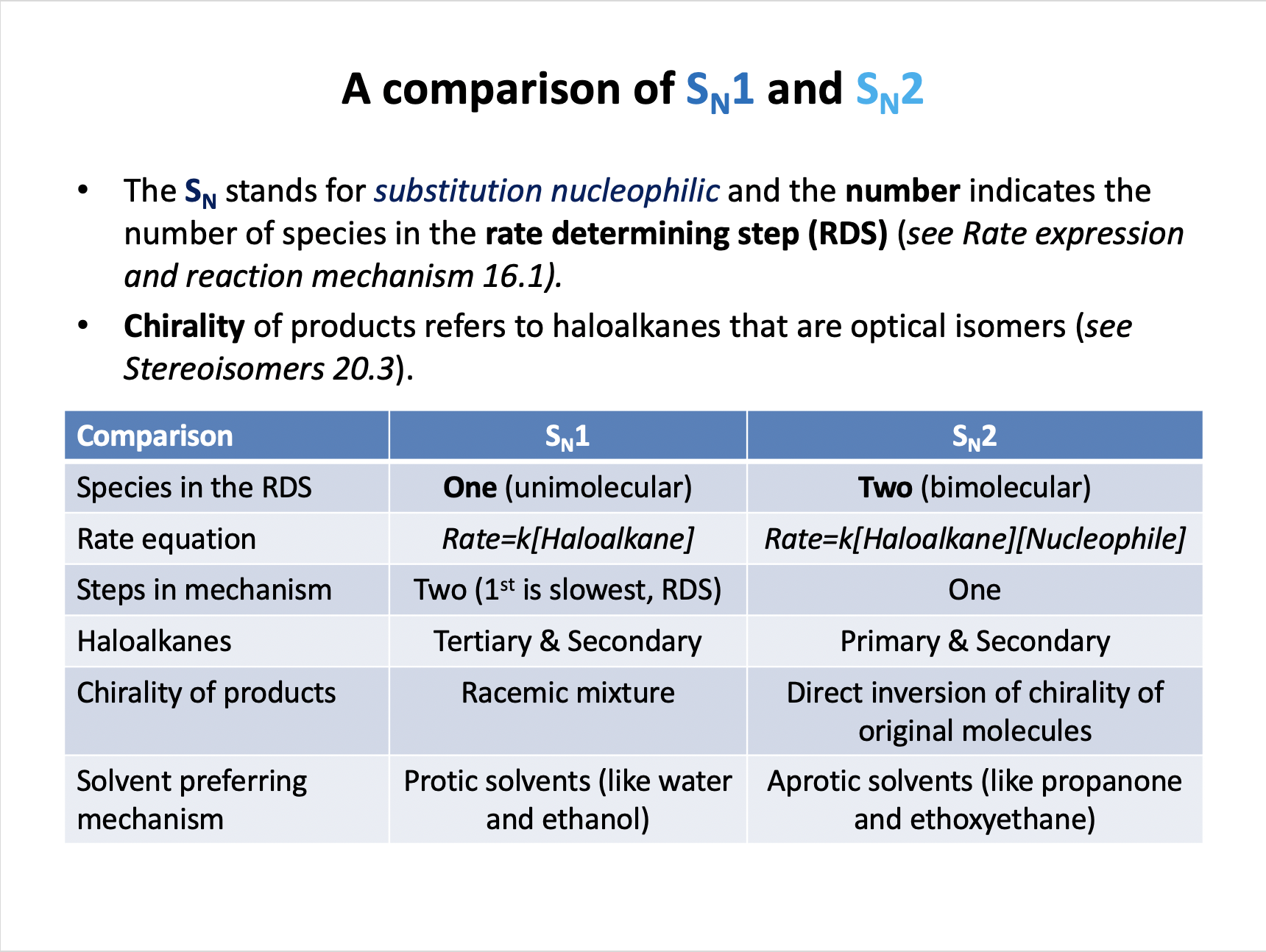
Which compound is most likely to react via an SN1 mechanism when reacted with sodium hydroxide in aqueous solution?
Nucleophilic substitution SN1 is favoured by tertiary haloalkanes, like (CH3)3CCl.
Primary haloalkanes (CH3CH2Cl and CH3CH2CH2Cl) favour SN2 substitution.
Benzene derivatives like chlorobenzene (C6H5Cl) favour electrophilic substitution as a mechanism for reaction.
Therefore the correct answer is (CH3)3CCl.
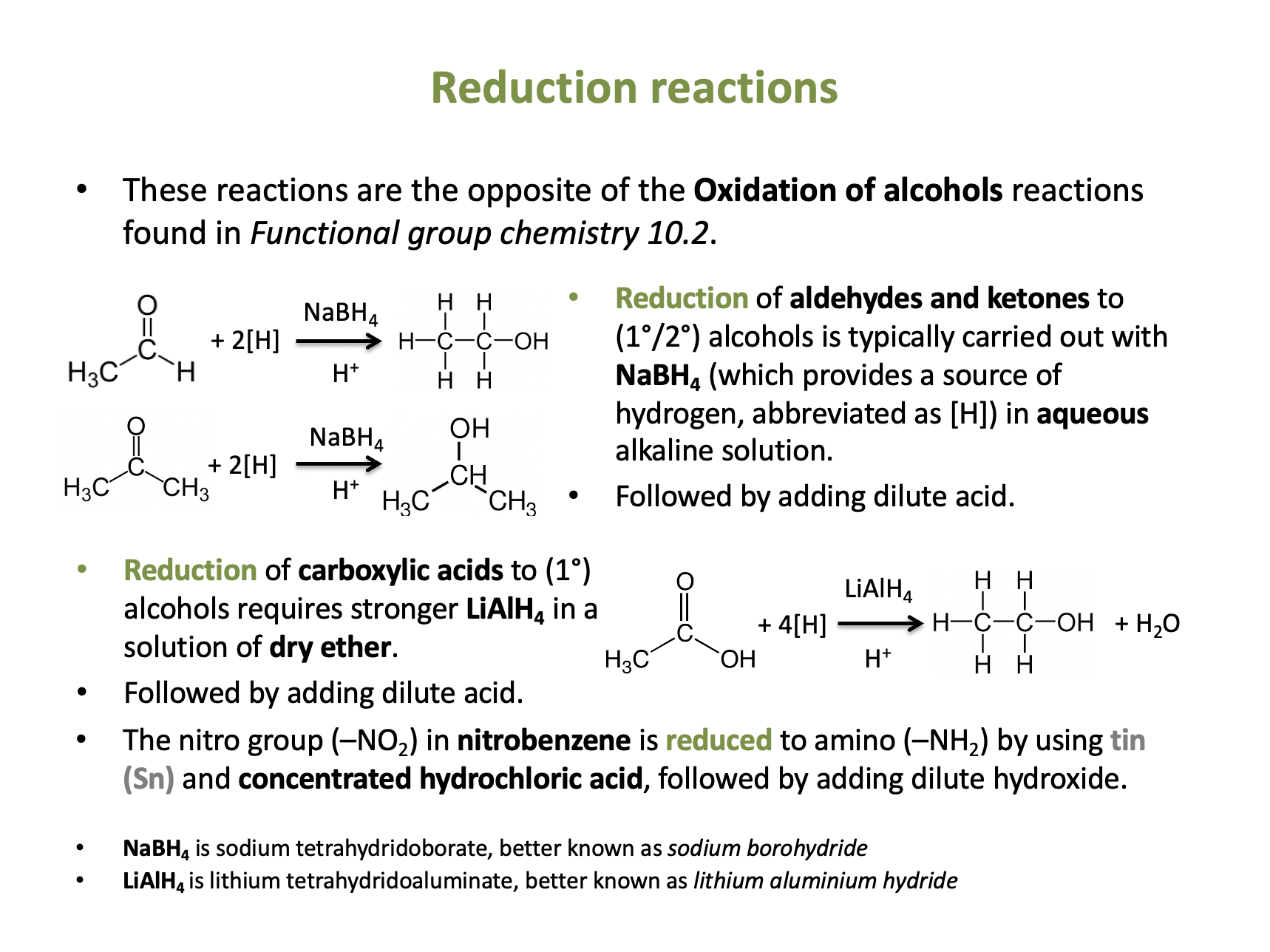
Which reagent will react with CH3CHO to produce a new organic product?
1: K2Cr2O7 / H+
2: NaBH4(aq) followed by reaction with H+
Aldehydes (CH3CHO is ethanal) can be oxidised to carboxylic acids using K2Cr2O7 / H+. They can also be reduced to primary alcohols using NaBH4(aq) followed by reaction with H+.
Therefore 1 and 2 is the correct answer.
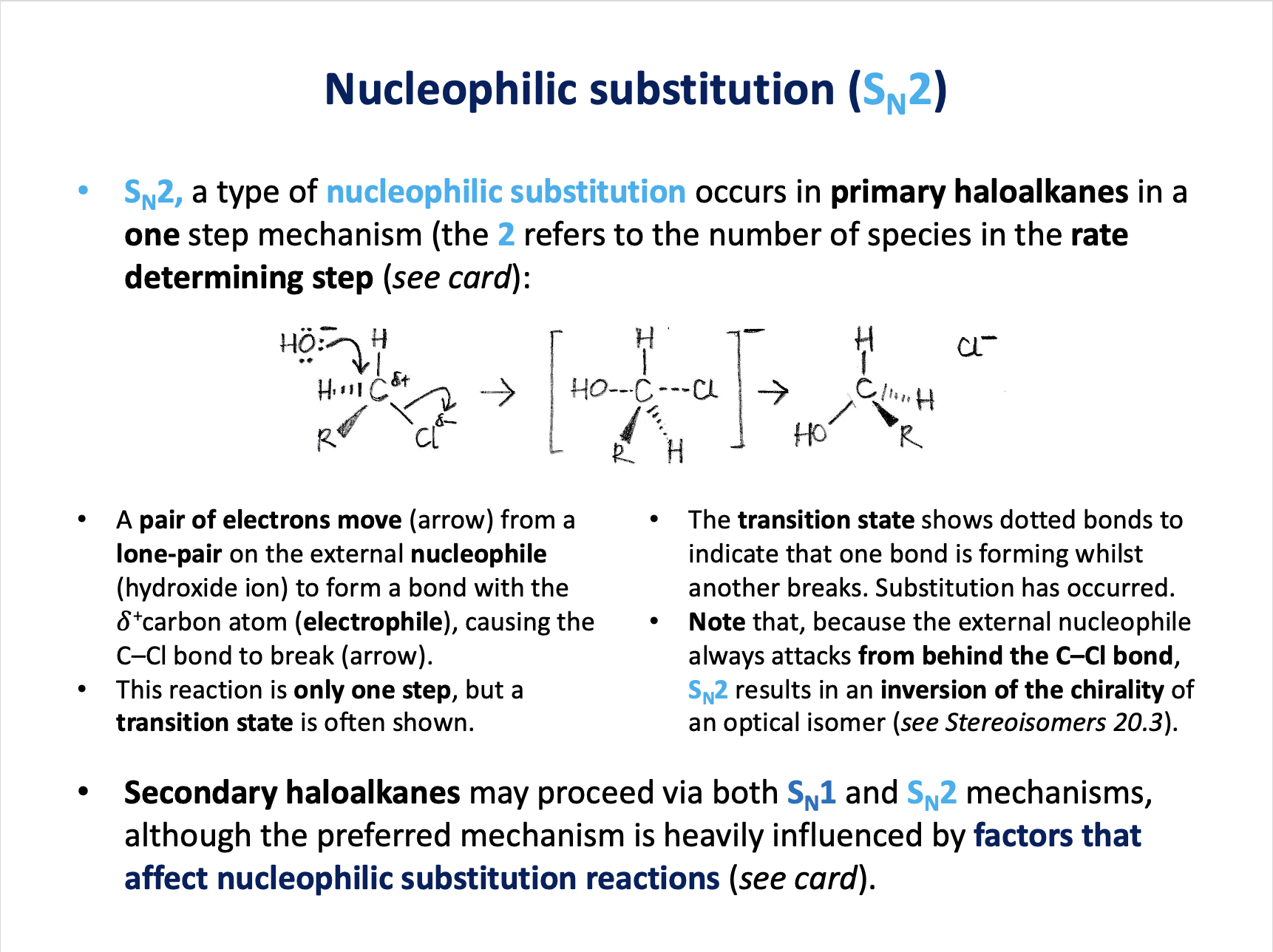
The diagram below represents a nucleophilic substitution mechanism.
Which of the following statements about this mechanism is/are correct?
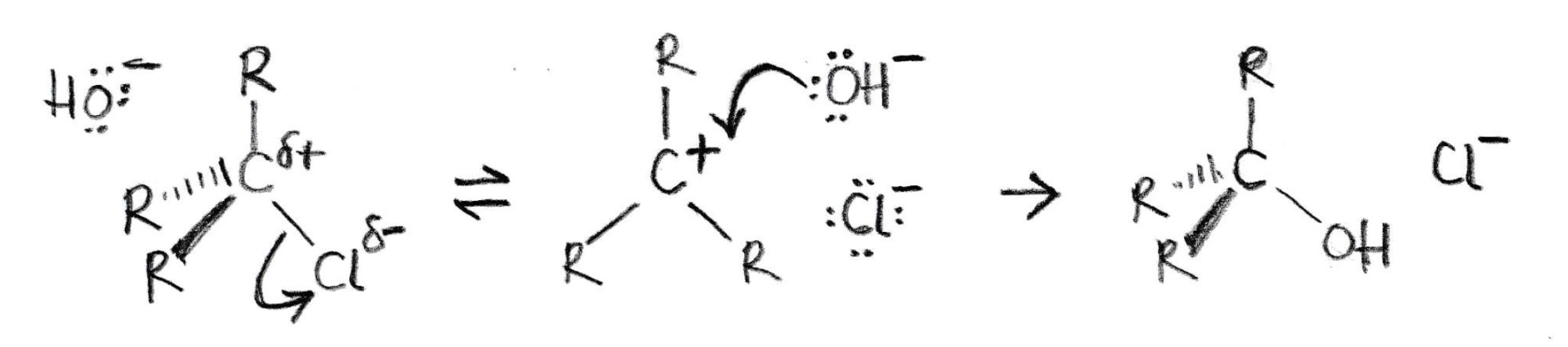
1: The OH− ion is a nucleophile
2: The scheme shows an SN2 mechanism
3: The OH− ion does not appear in the rate equation
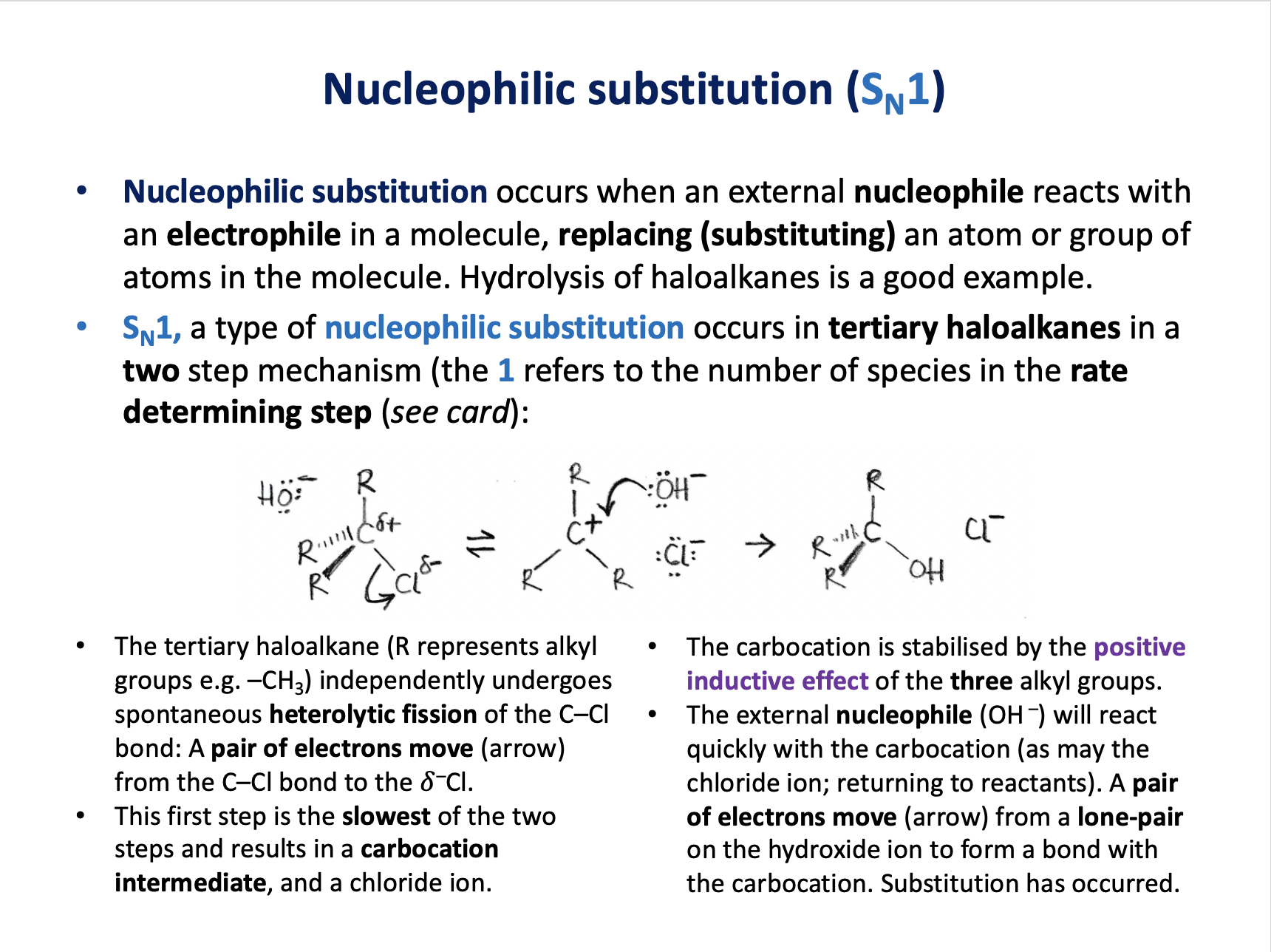
The mechanism shown is SN1 nucleophilic substitution.
The first step is the slowest step. The tertiary haloalkane (R represents alkyl groups e.g. –CH3) independently undergoes spontaneous heterolytic fission of the C–Cl bond.
Therefore only the haloalkane appears in the rate equation. The OH− ion does not appear in the rate equation because it is not involved in the slowest (rate-determining) step.
The OH− ion does behave as a nucleophile - donating a pair of electrons to form the C−OH bond.
Therefore 1 and 3 only is the correct answer.
Which of the following cannot be obtained by the oxidation of propan-1-ol under appropriate conditions?
Using acidified potassium dichromate, primary alcohols (propan-1-ol) can be oxidised to aldehydes (propanal) using distillation, or to carboxylic acids (propanoic acid) using reflux.
Alcohols can also undergo combustion to produce carbon dioxide and water - which is complete oxidation.
Primary alcohols cannot be oxidised to ketones (propanone). Ketones are produced from secondary alcohols.
Therefore Propanone is the correct answer.
Which compound will react the fastest when undergoing a nucleophilic substitution reaction with aqueous sodium hydroxide solution?
Tertiary haloalkanes ((CH3)3CCl and (CH3)3CBr)) react faster than primary haloalkanes, as they react via the SN1 mechanism (which tends to be faster than SN2).
Bromoalkanes react faster than chloroalkanes because the C−Br bond is longer and weaker than the C−Cl bond.
Therefore (CH3)3CBr is the correct answer.
Paper 1
Core (SL&HL): Organic chemistry core (SL and HL) paper 1 questions
AHL (HL only): Organic chemistry AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Organic chemistry core (SL & HL) paper 2 questions
AHL (HL only): Organic chemistry AHL (HL only) paper 2 questions
How much of Types of organic reactions and synthetic routes have you understood?















 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn