 A heat engine converts thermal energy into mechanical work.
A heat engine converts thermal energy into mechanical work.
Key Concepts
Heat is transferred from a thermal energy store at high temperature to a thermal energy sink of lower temperature. This movement enables work to be done on the surroundings in what is referred to as a heat engine.

\(Q_\textrm{H} =W_\textrm{out}+Q_\textrm{C}\)
 A refrigerator or heat pump is the opposite. Work is done on the gas to transfer heat from a low temperature energy store to a high temperature store.
A refrigerator or heat pump is the opposite. Work is done on the gas to transfer heat from a low temperature energy store to a high temperature store.
\(W_\textrm{in}+Q_\textrm{C}=Q_\textrm{H}\)
Change in entropy is defined as the ratio of the heat added to a system to the temperature at which it is transferred:
\(\Delta S={\Delta Q\over T}\)
- \(\Delta S\) is change in entropy (JK-1)
- \(\Delta Q\) is change in heat (J), where \(\Delta Q>0\) for heat added to the gas
- \(T\) is absolute temperature (K)
If entropy is constant, the process is reversible and theoretical only. If entropy increases, the process is irreversible and possible.
How much of Second law have you understood?


 The second law of thermodynamics can be expressed in three ways.
The second law of thermodynamics can be expressed in three ways.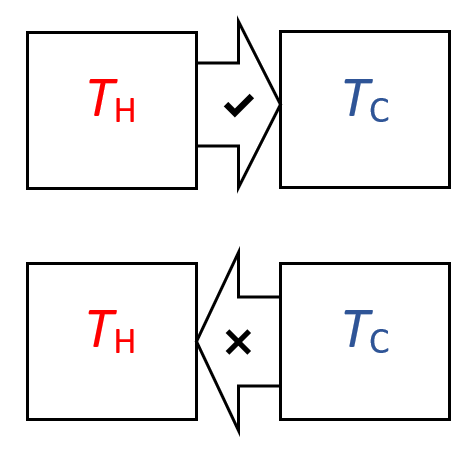 Let's consider the transfer of heat from a high temperature thermal energy store to a low temperature thermal energy sink:
Let's consider the transfer of heat from a high temperature thermal energy store to a low temperature thermal energy sink: .png) Some philosophically minded physicists suggest that ever-increasing entropy will lead to a maximum value. This would bring about the heat death of the universe.
Some philosophically minded physicists suggest that ever-increasing entropy will lead to a maximum value. This would bring about the heat death of the universe. Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn