
To avoid needlessly long descriptions, we can use diagrams to show the processes that take place in a container of gas.
Key Concepts
A graph of pressure vs volume (\(pV\) diagram) enables us to show all possible types of process because of the interrelationship between pressure, volume and temperature in the ideal gas law: \(pV\propto T\)
We can use the first law to consider the changing heat, internal energy and work done: \(Q=\Delta U+p\Delta V\)
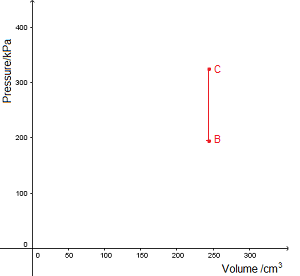 An isovolumetric process takes place at constant volume. This is represented by a vertical line (isochore) on a \(pV\) diagram.
An isovolumetric process takes place at constant volume. This is represented by a vertical line (isochore) on a \(pV\) diagram.
In this example, \(\textrm{B}\to\textrm{C}\) represents an increase in pressure and temperature.
\(p\propto T\)
- \(p\) is pressure (Pa)
- \(T\) is temperature (K)
\({p_1\over T_1}={p_2\over T_2}\)
Since internal energy is increasing, heat must be gained by the gas. No work is done.
How much of Processes have you understood?


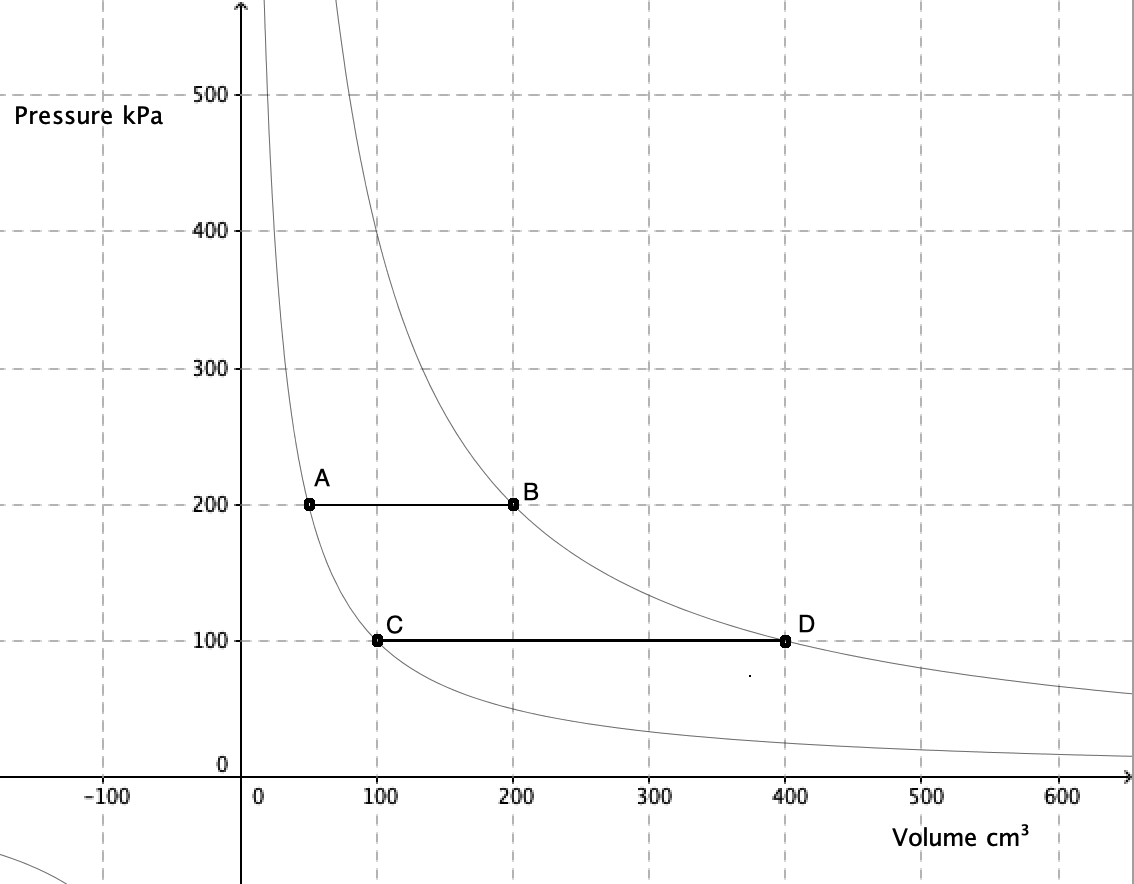
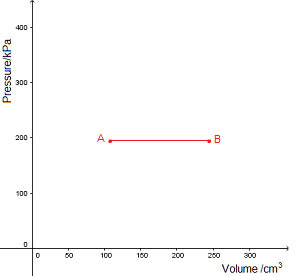 An isobaric process takes place at constant pressure. This is represented by a horizontal line (isobar) on a
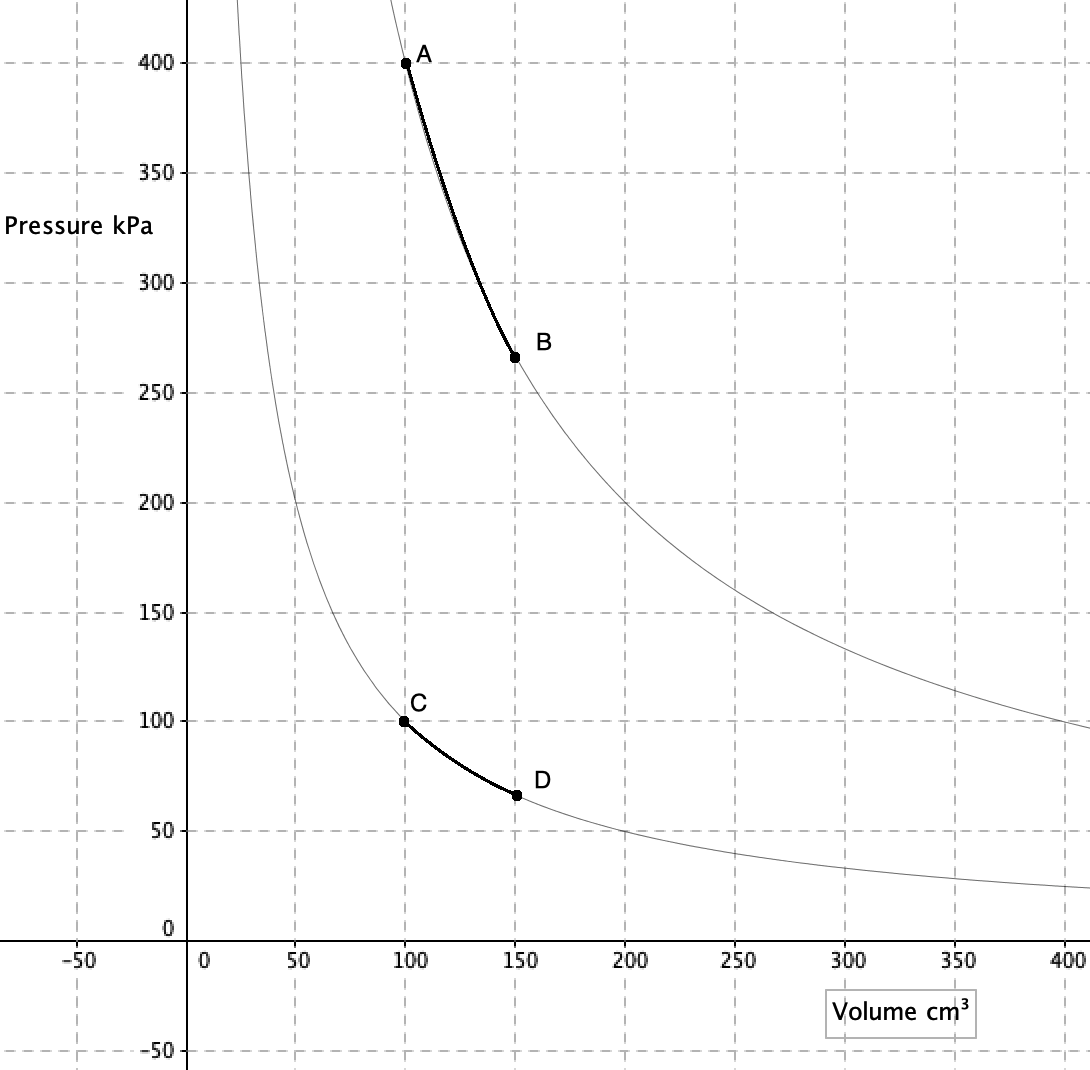
An isobaric process takes place at constant pressure. This is represented by a horizontal line (isobar) on a 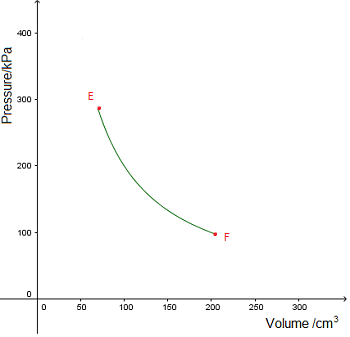 n isothermal process takes place at constant temperature. This is represented by a line of inverse proportion (isotherm) on a
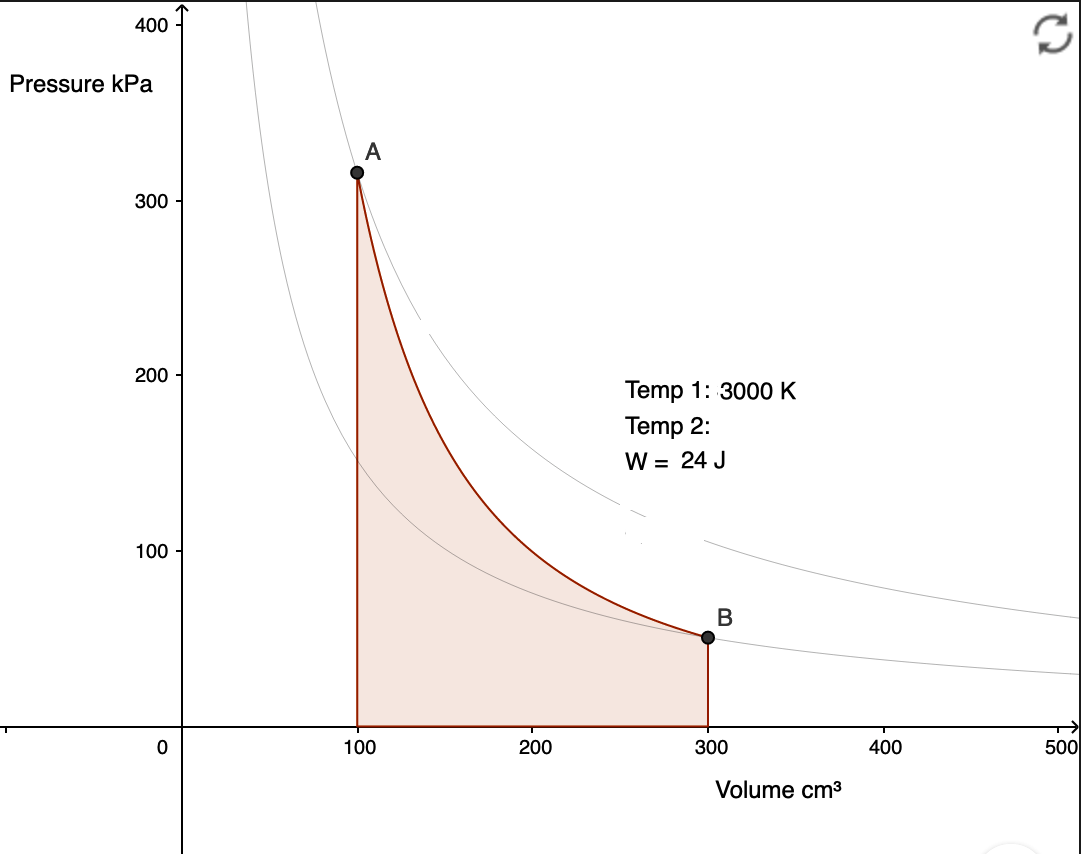
n isothermal process takes place at constant temperature. This is represented by a line of inverse proportion (isotherm) on a 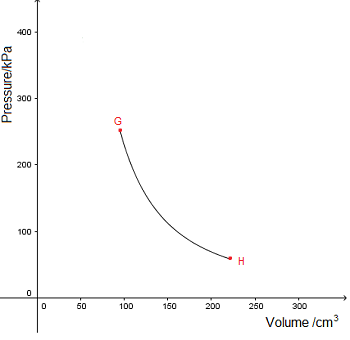 No heat is gained or lost during an adiabatic process. This is represented by an adiabat on a
No heat is gained or lost during an adiabatic process. This is represented by an adiabat on a 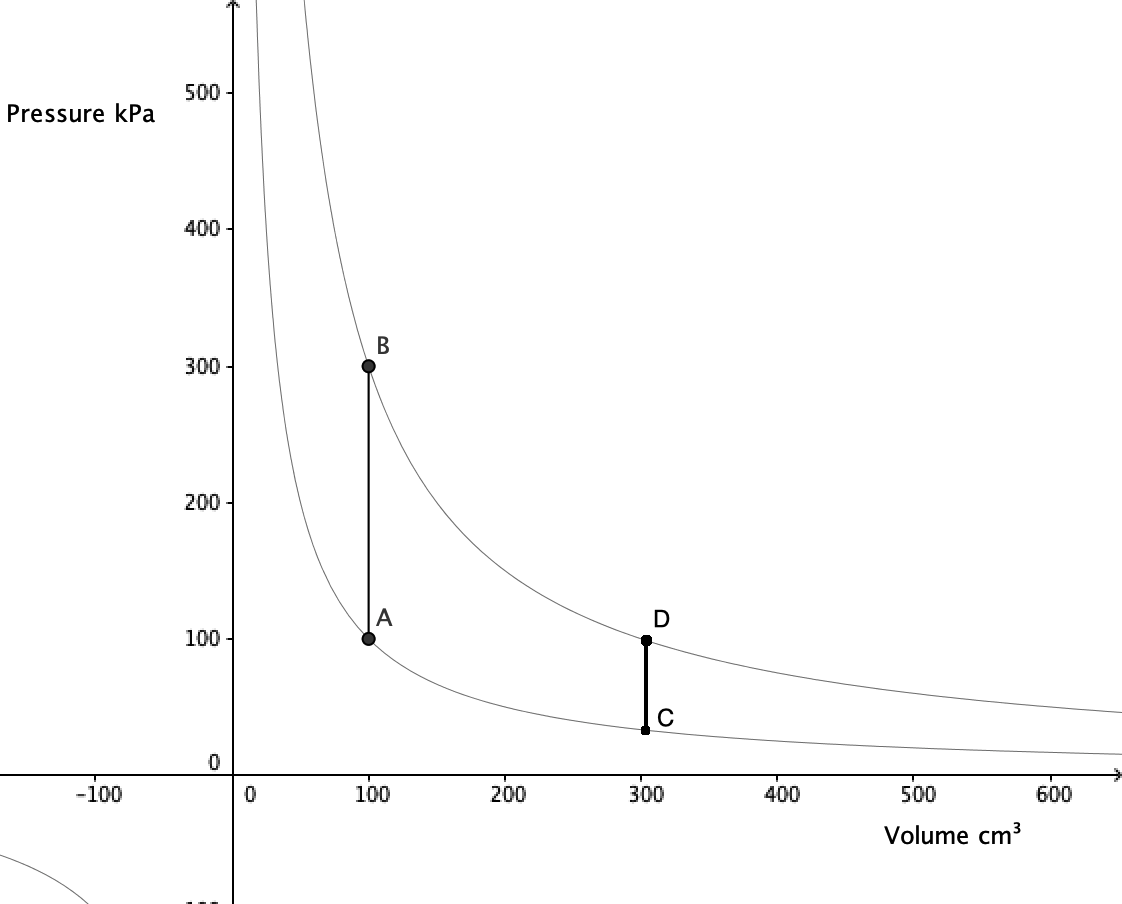
 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn