 A cyclic process is a series of transformations that take the gas back to its original state. These form a closed loop on a \(pV\) diagram.
A cyclic process is a series of transformations that take the gas back to its original state. These form a closed loop on a \(pV\) diagram.
Key Concepts
A simple cyclic process involves:
- Isobaric expansion - work is done on the surroundings
- Isovolumetric cooling
- Isobaric compression - work is done on the gas
- Isovolumetric heating
Let's consider the cycle for a heat engine.
Since \(W=p\Delta V\), the area between a line on the \(pV\) diagram and the \(V\) axis gives work done. The net work done during a cyclic process is given by the enclosed area.
We can show the net work done using a Sankey diagram.
The dimensionless quantity, thermal efficiency, of any mechanical process is defined as the ratio of the useful work done to the total energy input:
\(\eta={\textrm{useful work done}\over \textrm{energy input}}\)
For a heat engine:
- Energy input is \(Q_\textrm{H}\)
- Work done is \(W_\textrm{out}=Q_\textrm{H}-Q_\textrm{C}\)
\(\eta={Q_\textrm{H}-Q_\textrm{C}\over Q_\textrm{H}}=1-{Q_\textrm{C}\over Q_\textrm{H}}\)
How much of Cyclic processes have you understood?


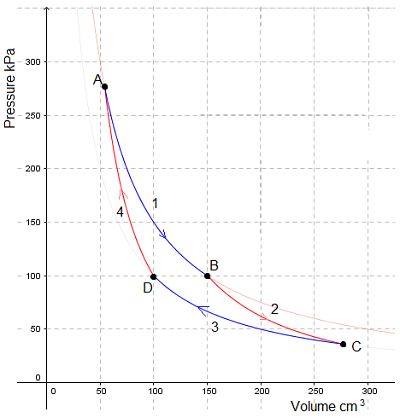 Isothermal expansion - work is done on the surroundings
Isothermal expansion - work is done on the surroundings
 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn