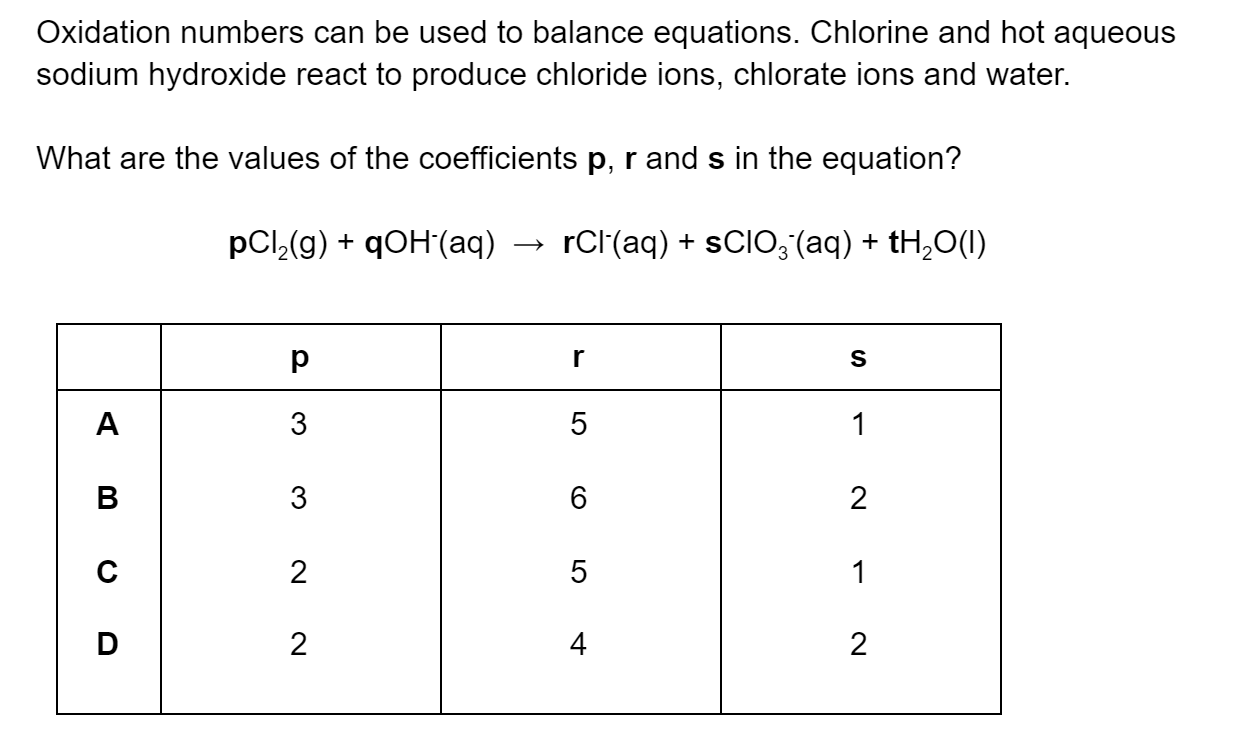
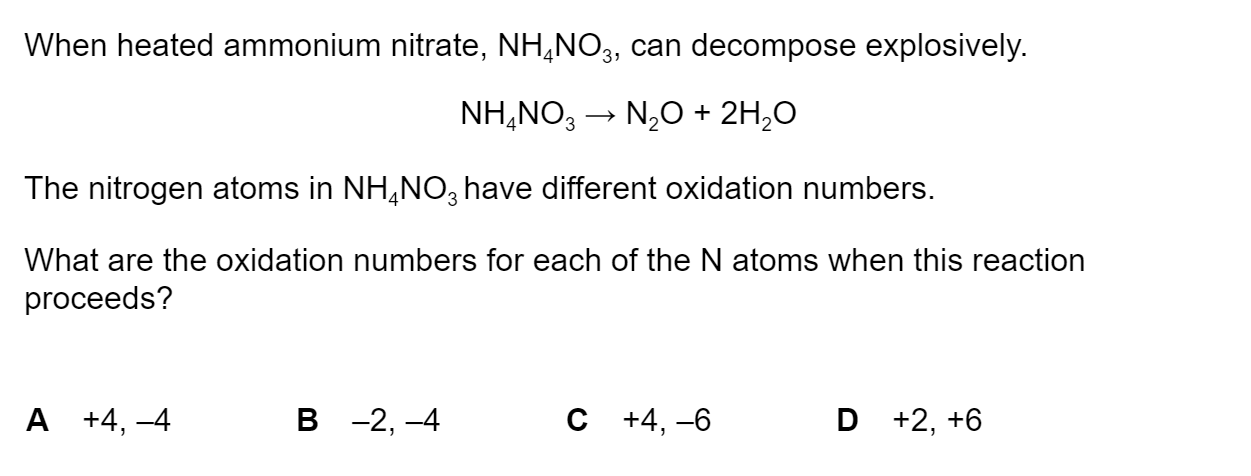
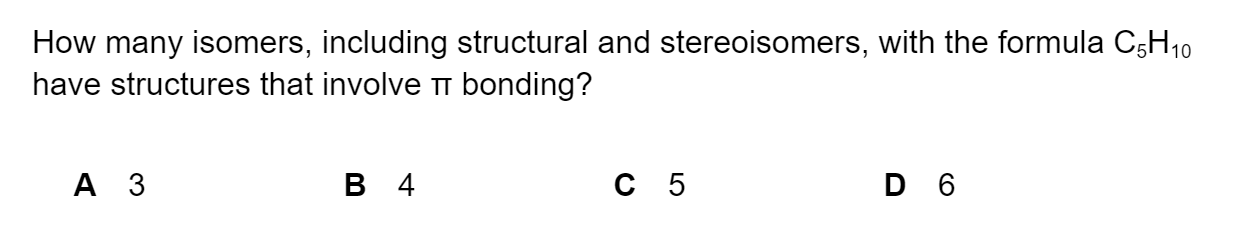
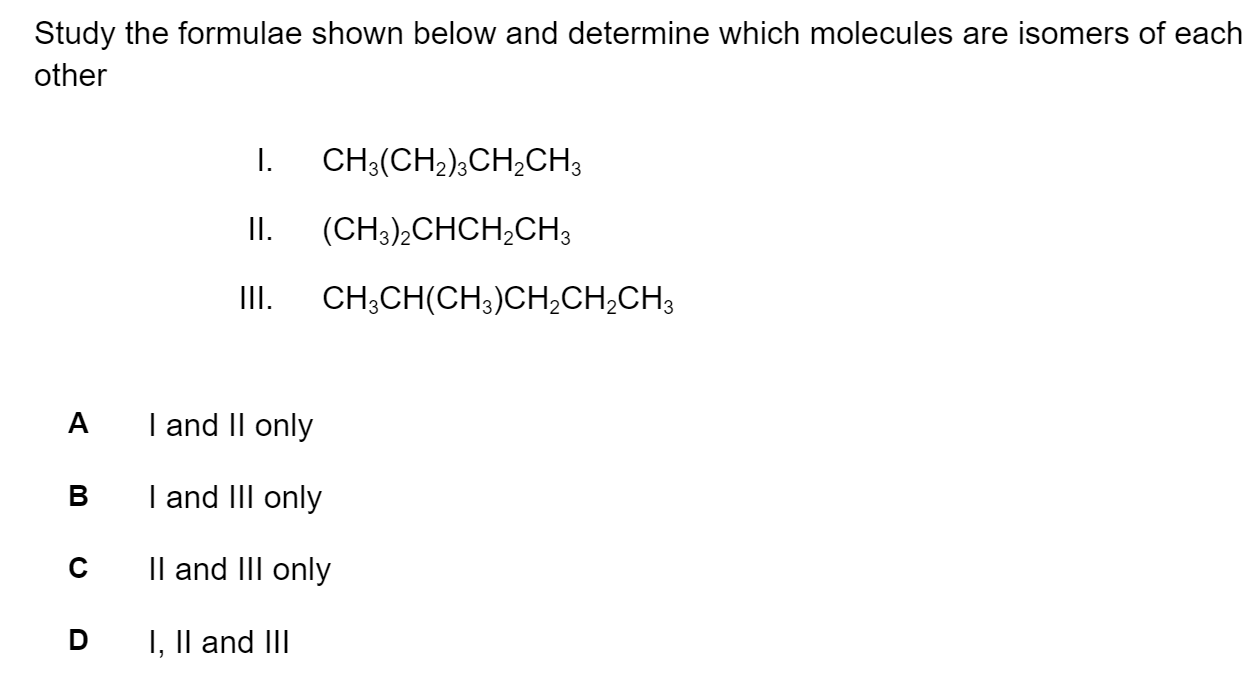
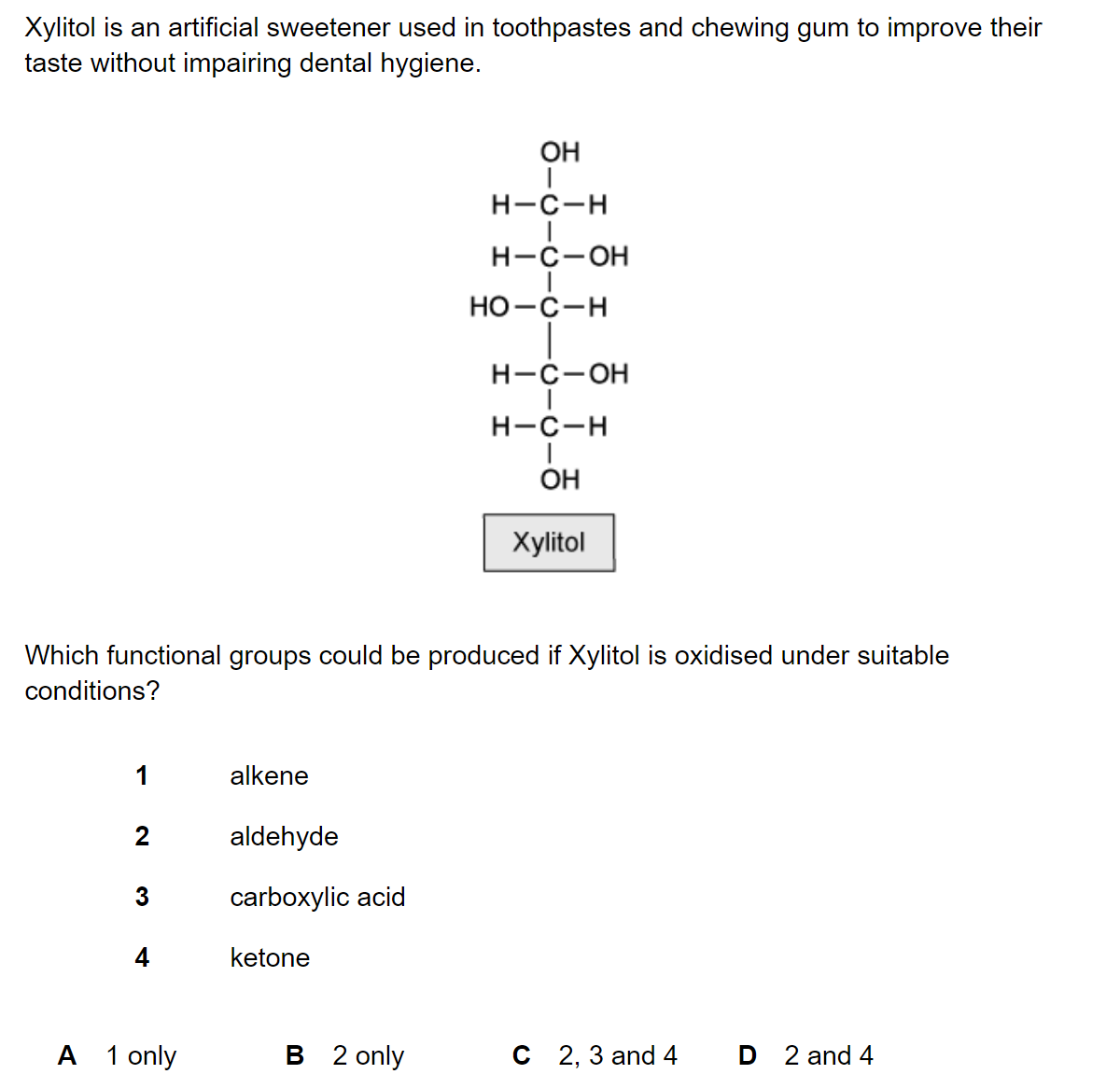
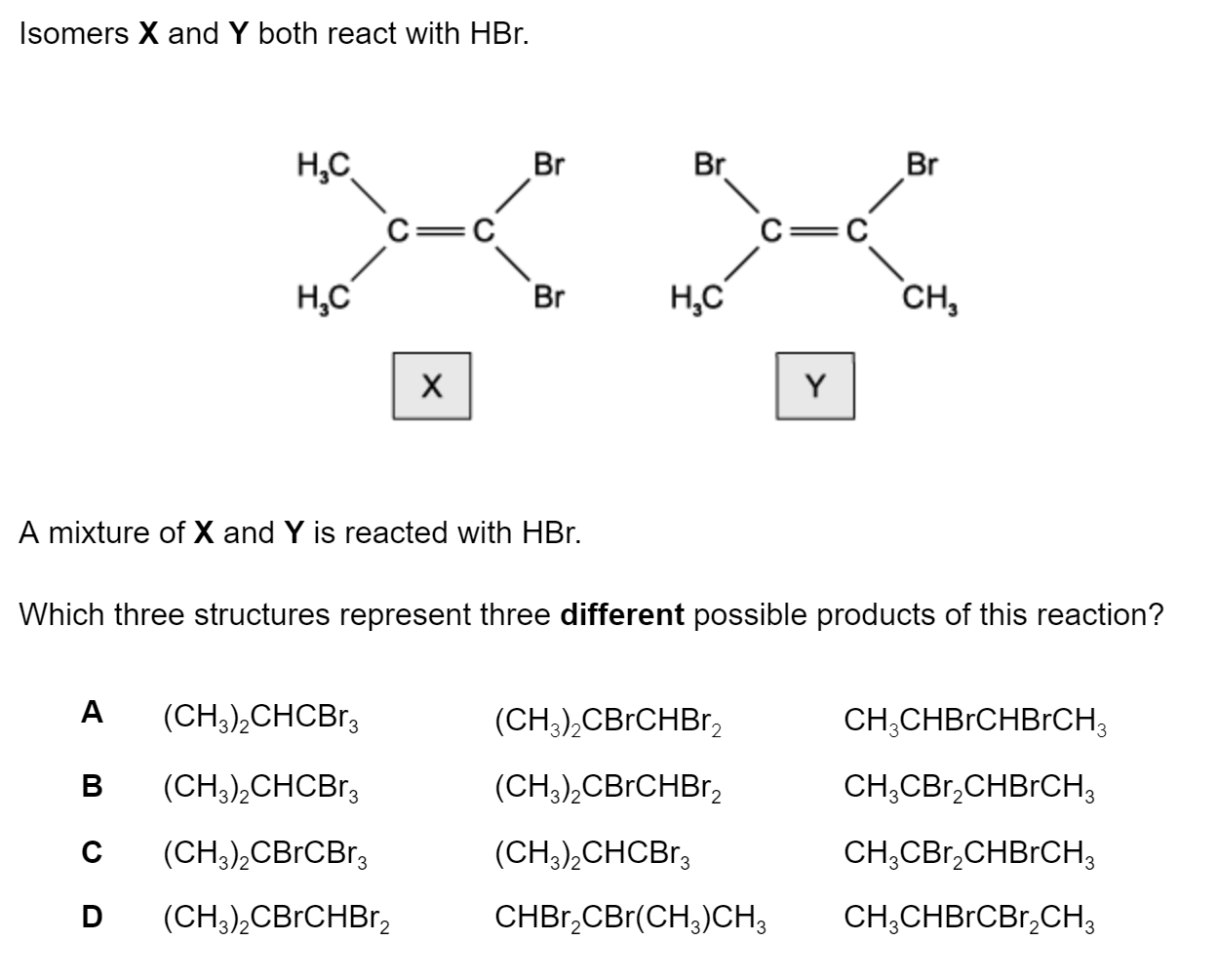
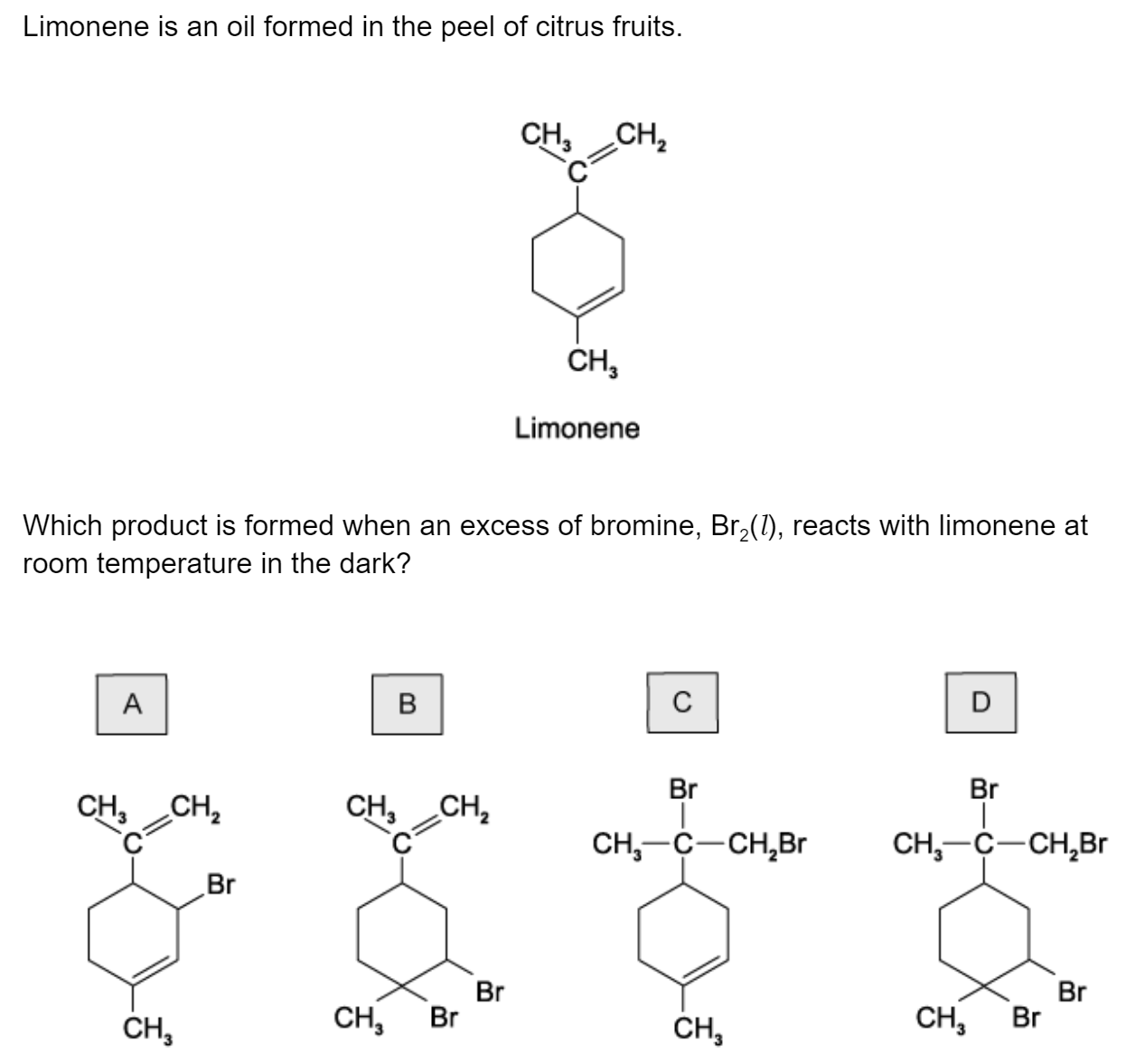
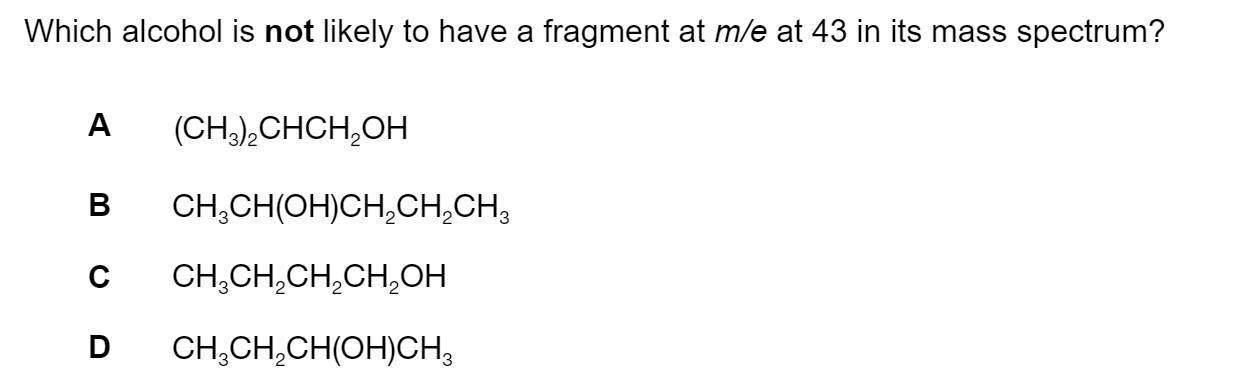
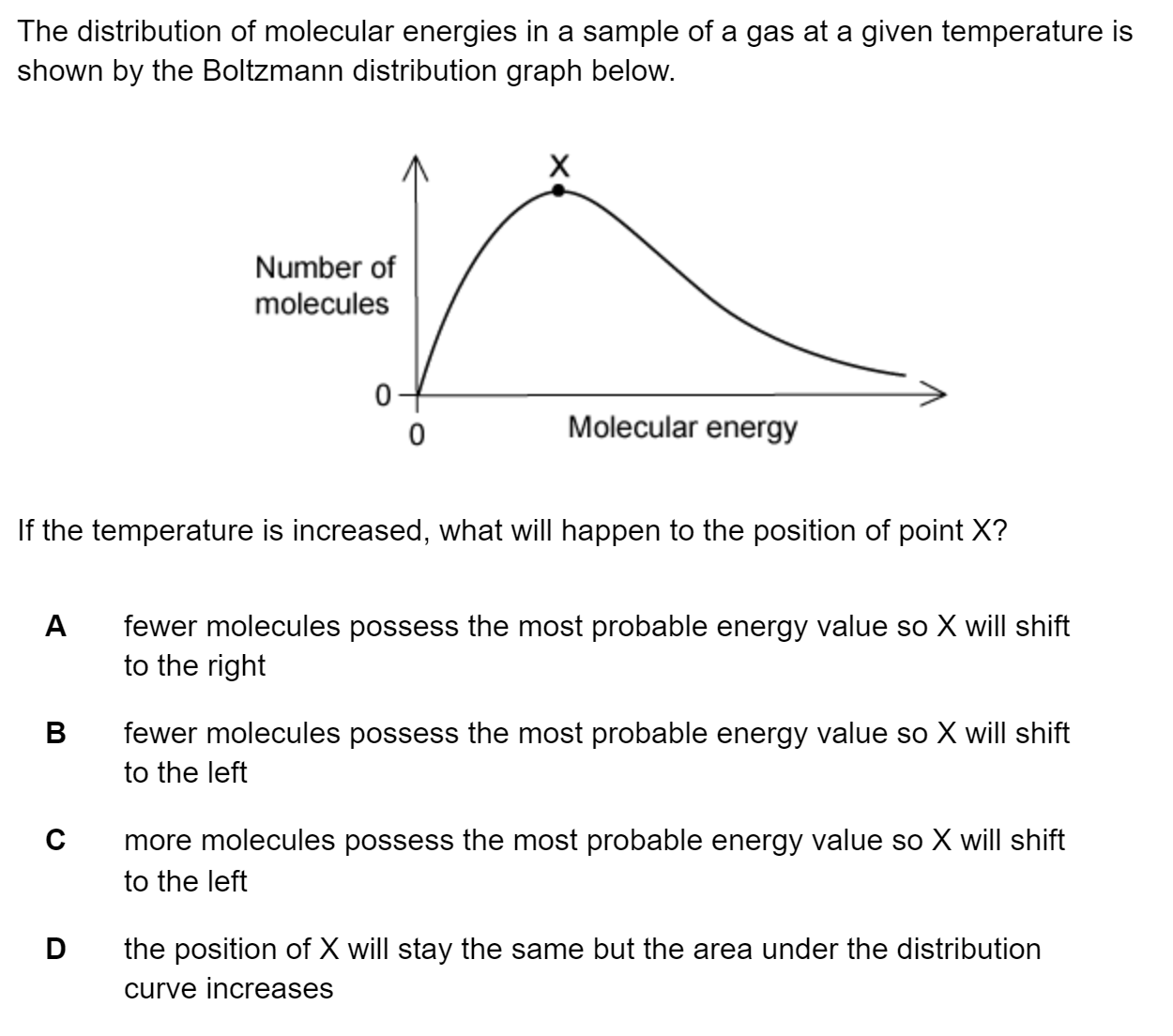
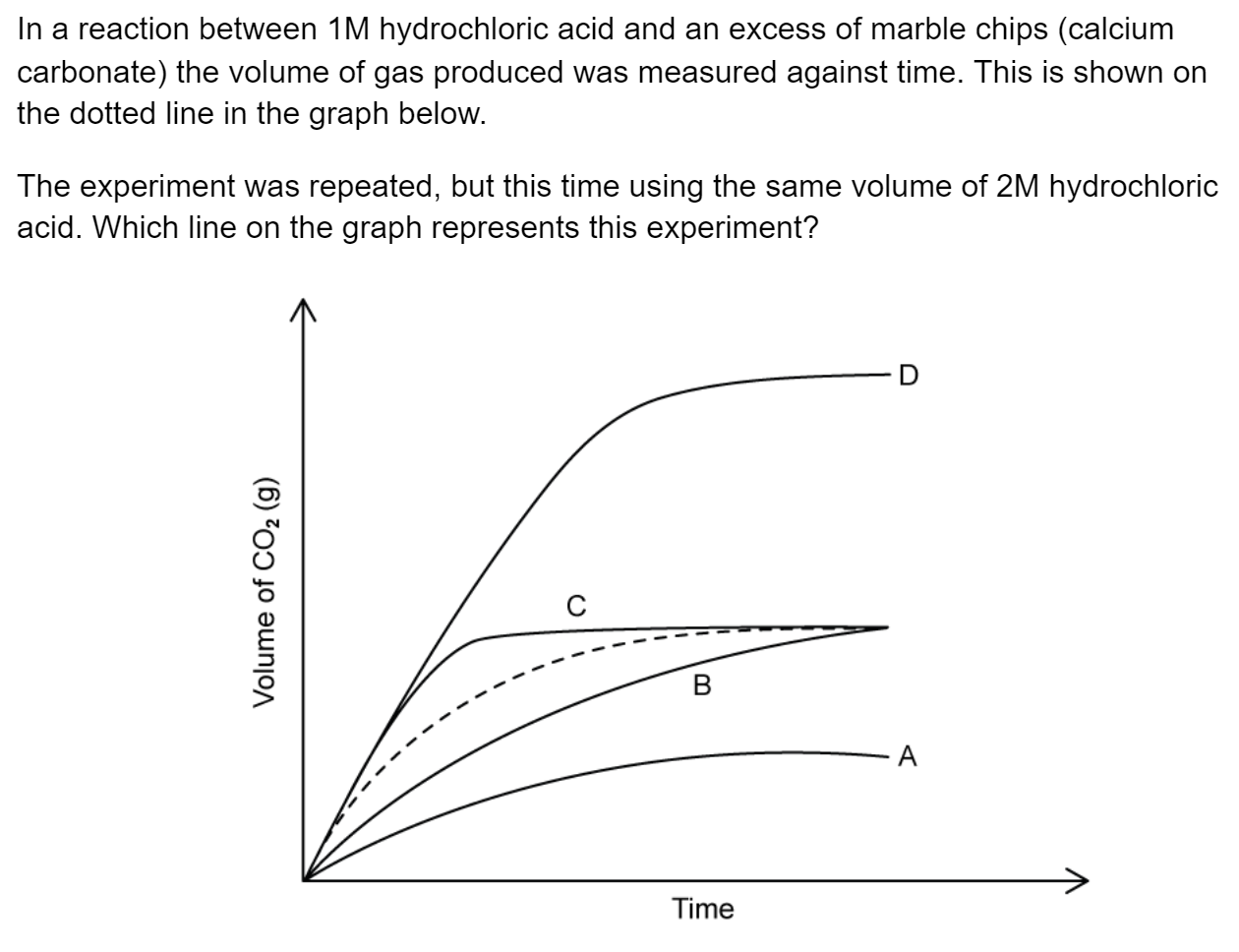
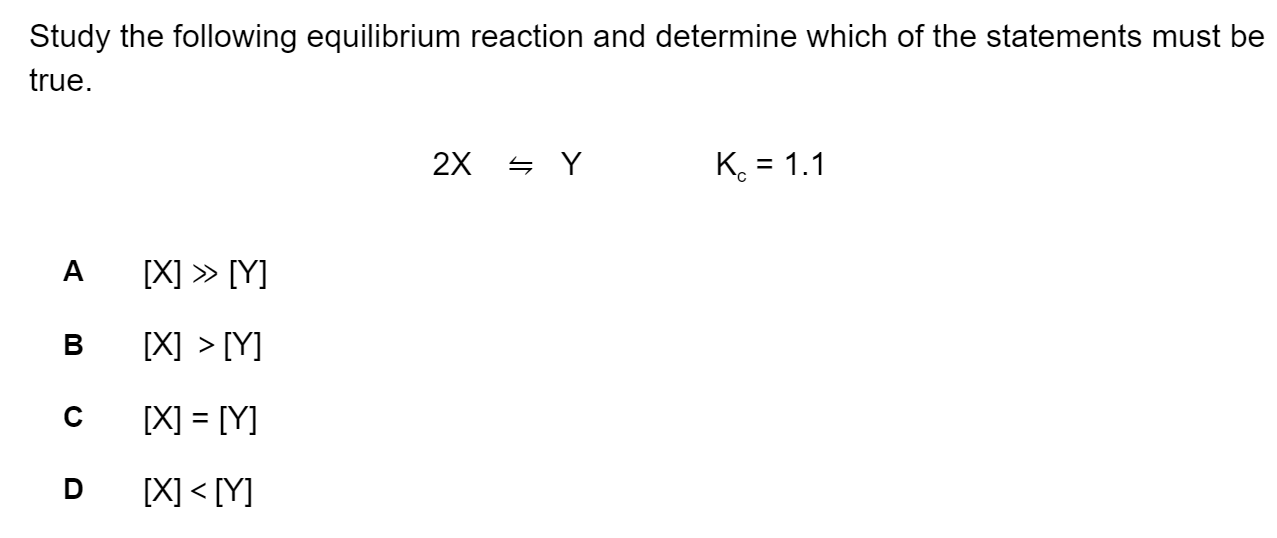
Question 1


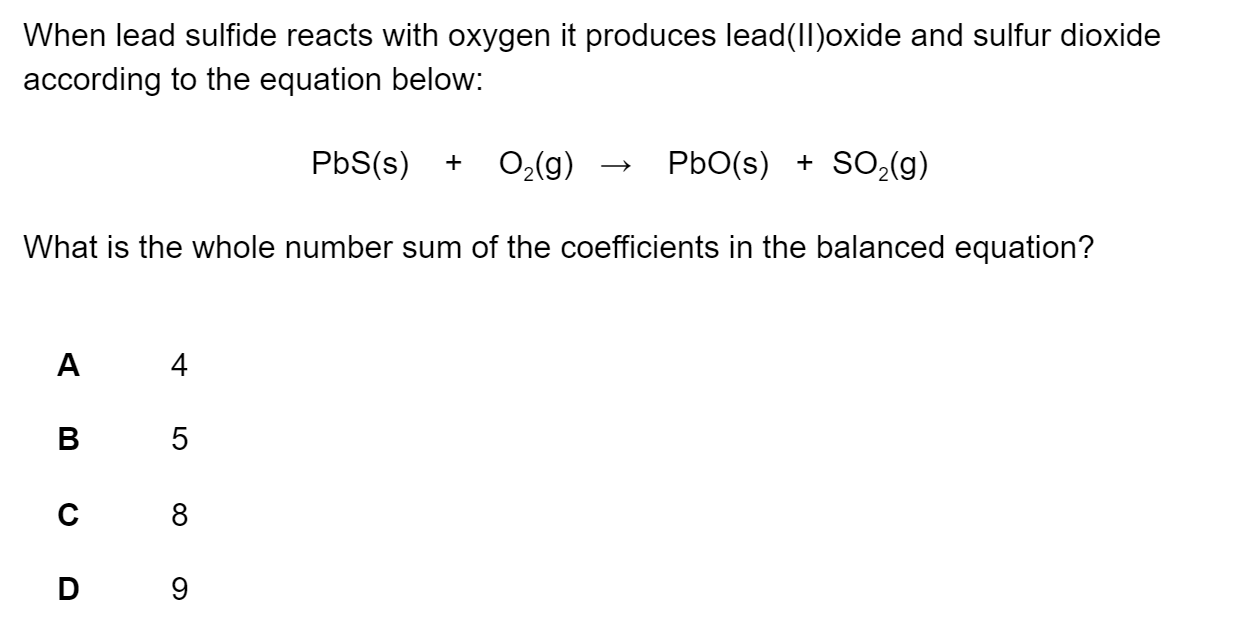


Electron configurations for atoms of different elements are shown below.
Which electron configuration represents the element with the largest first ionisation energy?
1s22s22p63s2
1s22s22p63s23p4
1s22s22p63s23p6
1s22s22p63s23p64s2
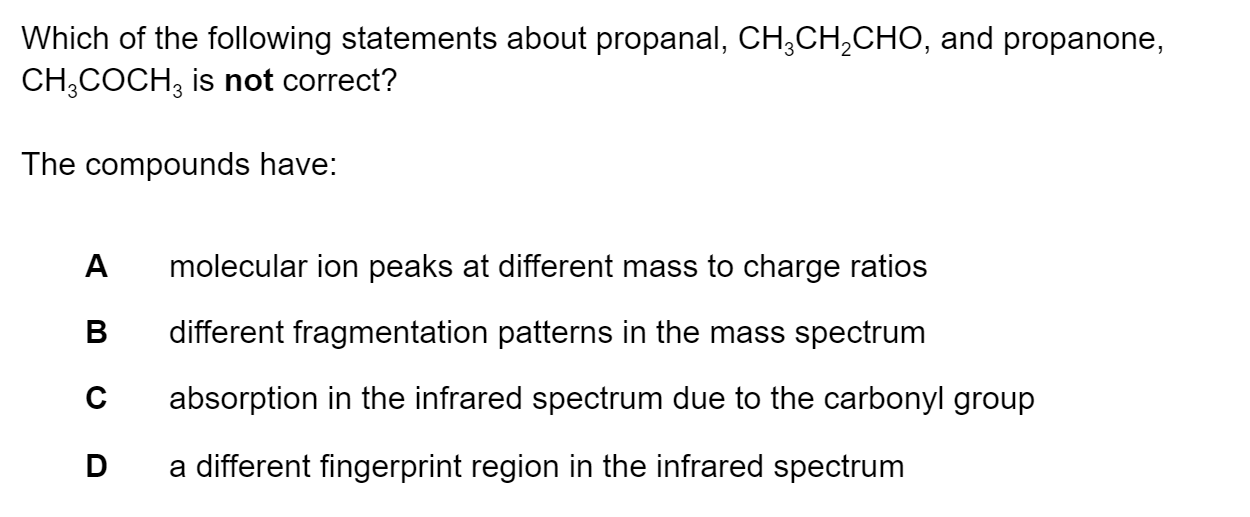
Which of the following statements about 2-methylpropan-2-ol, CH3C(CH3)(OH)CH3, are correct ?
I and II only
I and III only
II and III only
I, II and III
The following equation shows the dissociation equilibrium of PCl5.
PCl5(g) → PCl3(g) + Cl2(g)
The percentage yield of PCl3 varies with temperature.
At 160°C PCl3 yield is 13% and at 300°C yield is100%.
Which of the following rows is correct?
|
|
The reaction is |
Shape of PCl3 molecule |
|
A |
exothermic |
trigonal pyramidal |
|
B |
exothermic |
trigonal planar |
|
C |
endothermic |
trigonal pyramidal |
|
D |
endothermic |
trigonal planar |
Which of the following metals would have the highest melting point?
Na
Mg
Al
K
The correct order of increasing boiling points for the following compounds is
1-chlorobutane < butane < butan-1-ol
Butan-1-ol < 1-chlorobutane < butane,
Butane < 1-chlorobutane < butan-1-ol
Butan-1-ol < butane < 1-chlorobutane
Which equation below can represent both an enthalpy change of formation and combustion?
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
2Na(s) + ½O2(g) → Na2O(s)
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
CO(g) + C(s) → CO2(g)
Titanium occurs naturally as the mineral rutile, TiO2. One possible method of extraction of titanium is to reduce the rutile by heating with carbon.
TiO2(s) + 2C(s) → Ti(s) + 2CO(g)
The standard enthalpy changes of formation of TiO2(s) and CO(g) are –890 kJ mol-1 and –110.5 kJ mol-1 respectively.
What is the standard enthalpy change of the extraction of titanium?
+ 669 kJ mol–1
+ 779.5 kJ mol–1
– 779.5 kJ mol–1
– 669 kJ mol–1
In the gas phase, phosphorus pentachloride can be thermally decomposed into gaseous phosphorus trichloride and chlorine.
PCl5 → PCl3 + Cl2
The table below gives the relevant bond energies found in these compounds.
|
bond |
bond energy / kJ mol–1 |
|
P–Cl (in both chlorides) Cl–Cl |
|
What is the enthalpy change in the decomposition of the reaction?
The standard enthalpy change, ΔHӨ, for the following reaction is -246 kJ.
N2(g) + 3F2(g) → 2NF3(g)
The bond energy of N≡N is 945 kJ mol-1 and F–F is 159 kJ mol-1
What is the bond energy of the N–F bond?
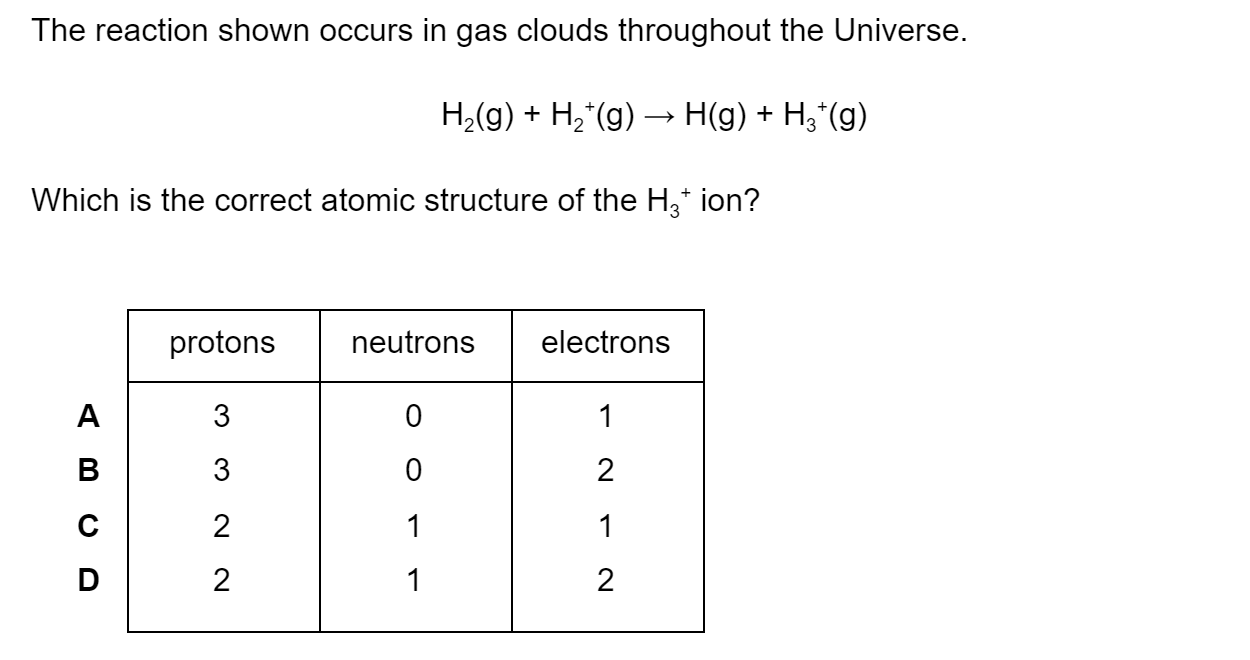
Some species may be classified as amphiprotic, some as amphoteric and some as both. Which of the following applies to HPO42−?
Amphiprotic but not amphoteric
Amphoteric but not amphiprotic
Amphiprotic and amphoteric
Neither amphiprotic nor amphoteric
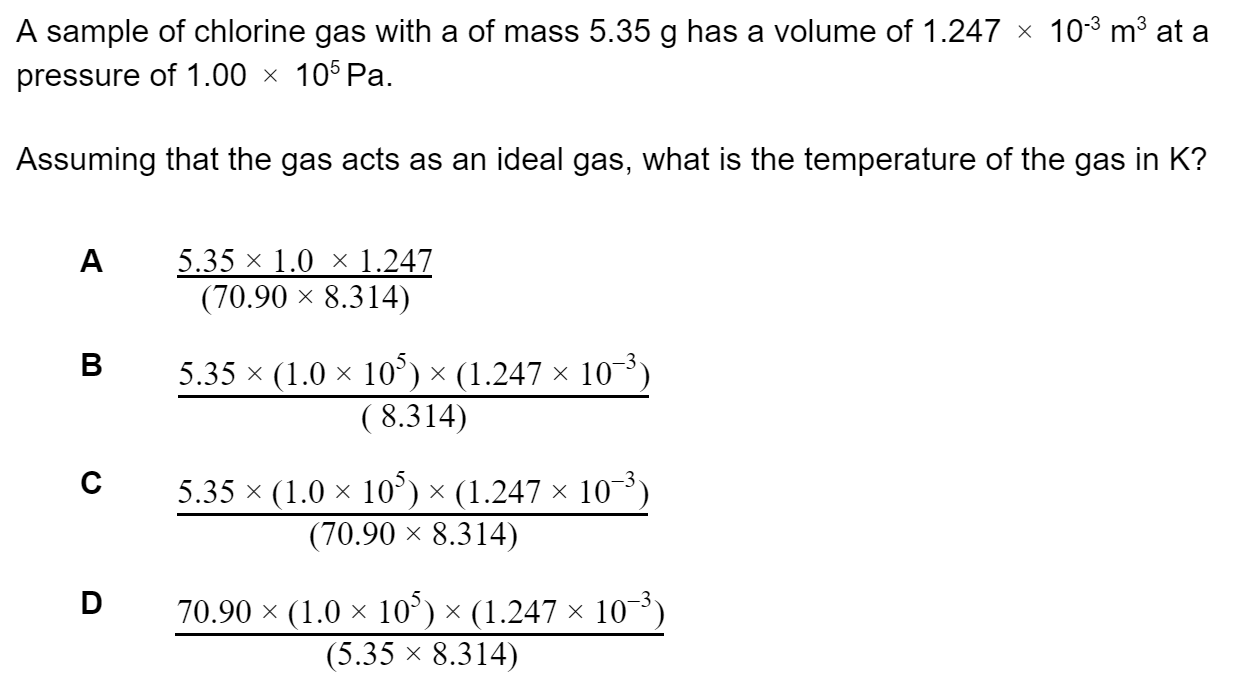
Determine which of the following solutions would be basic at 25 °C?
Kw = 1.0 × 10−14 mol2 dm-6
[H+] = 1.0 × 10−2 mol dm−3
[OH−] = 1.0 × 10−12 mol dm−3
solution of pH = 5.00
[H3O+] = 1.0 × 10−12 mol dm−3