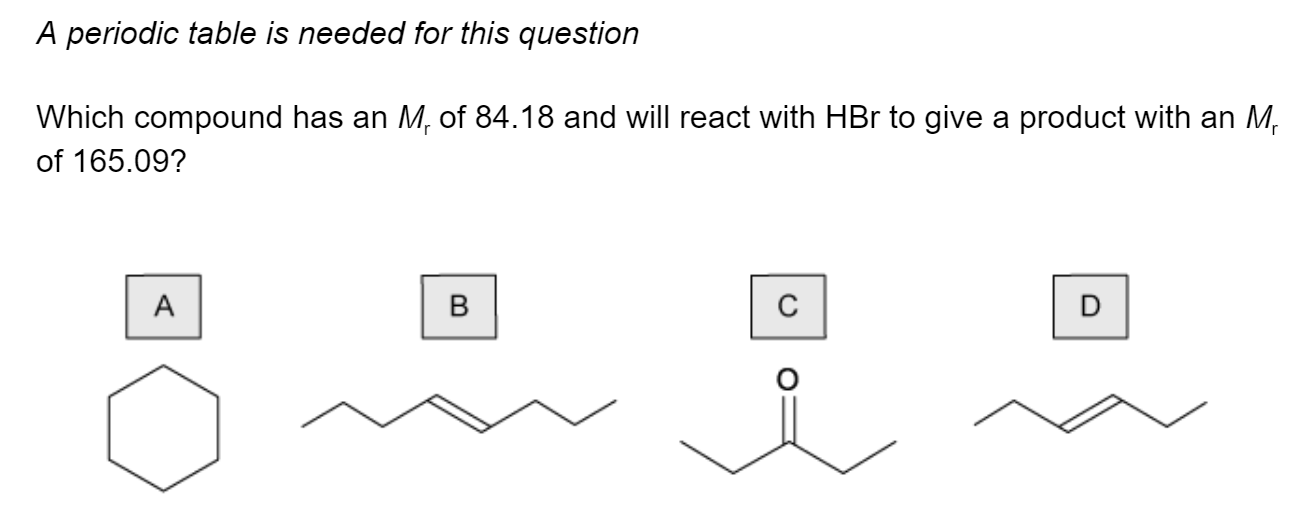
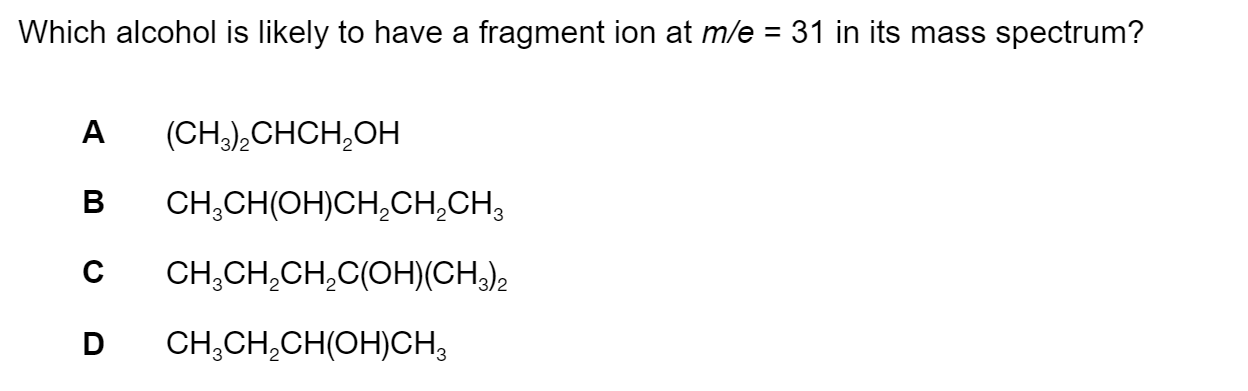
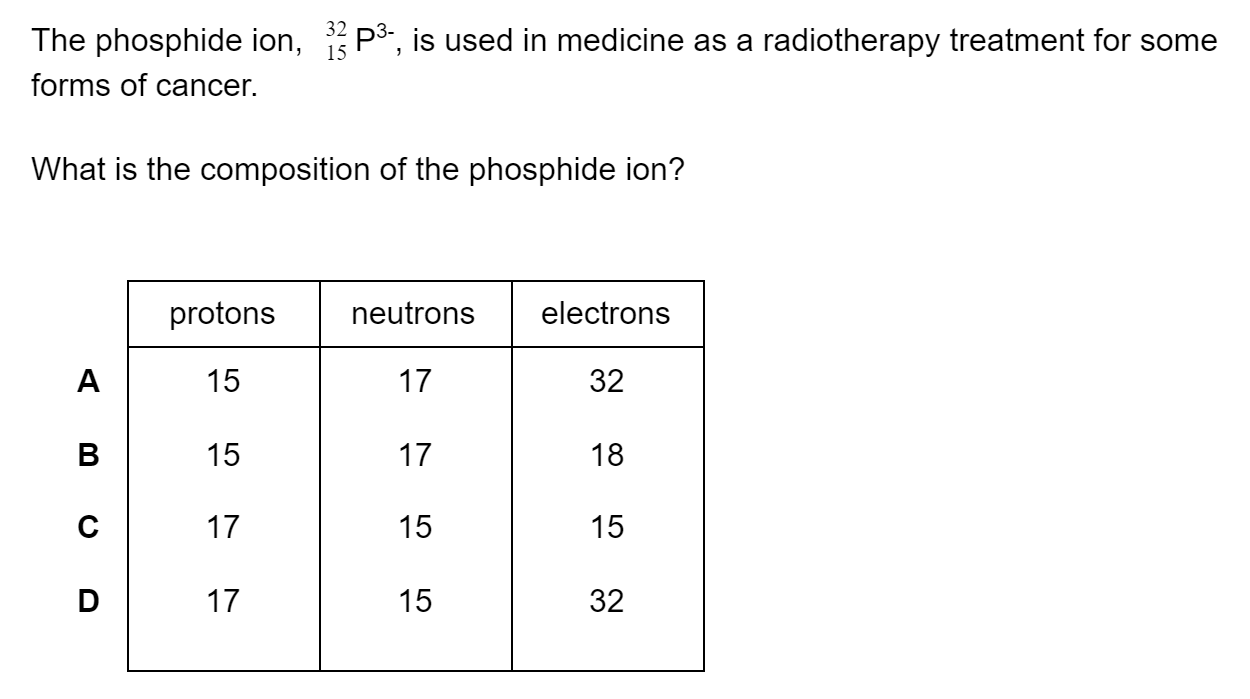
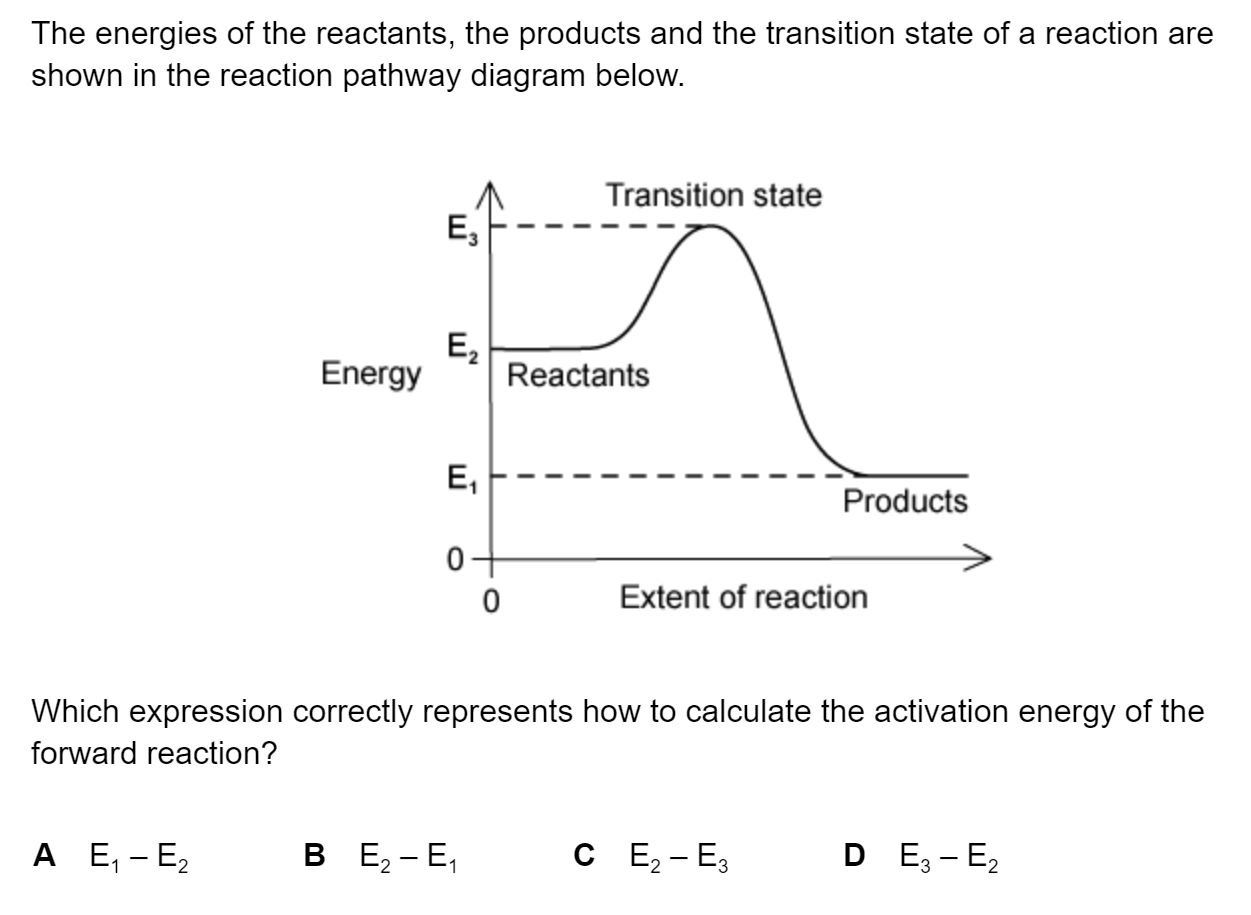
Question 1






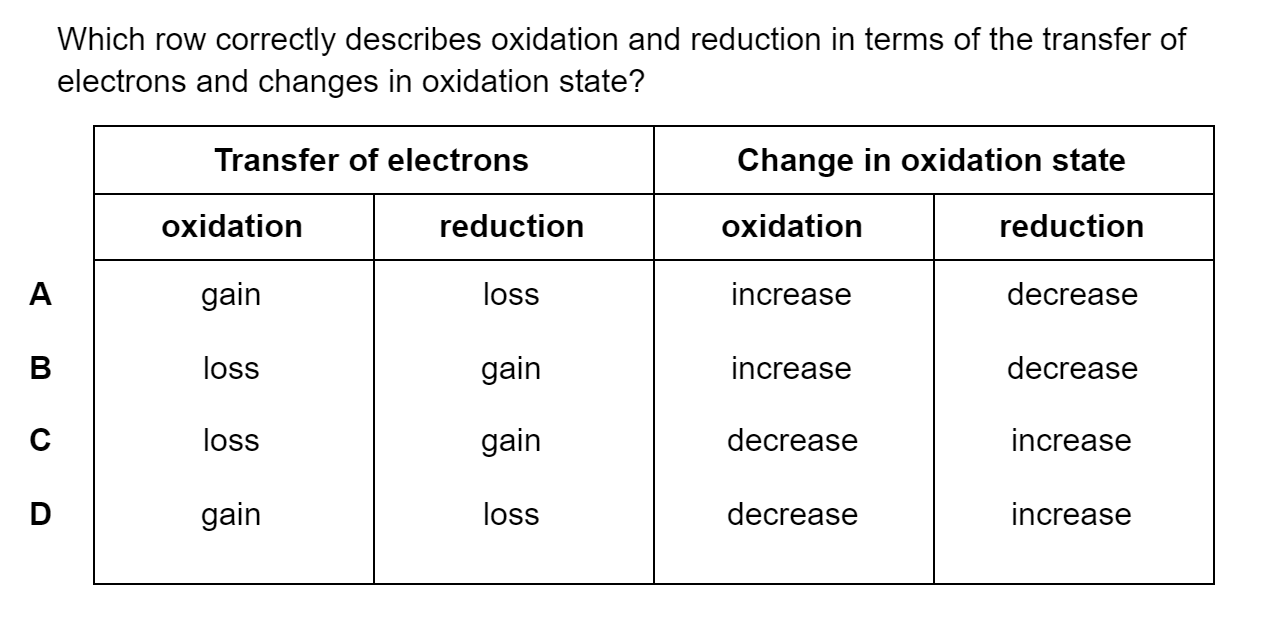
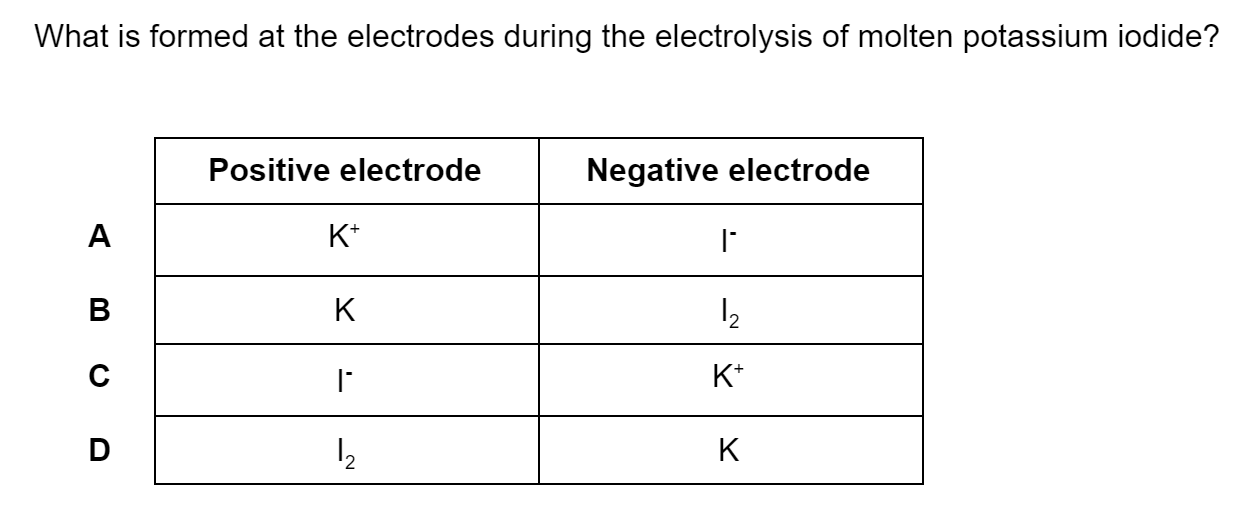
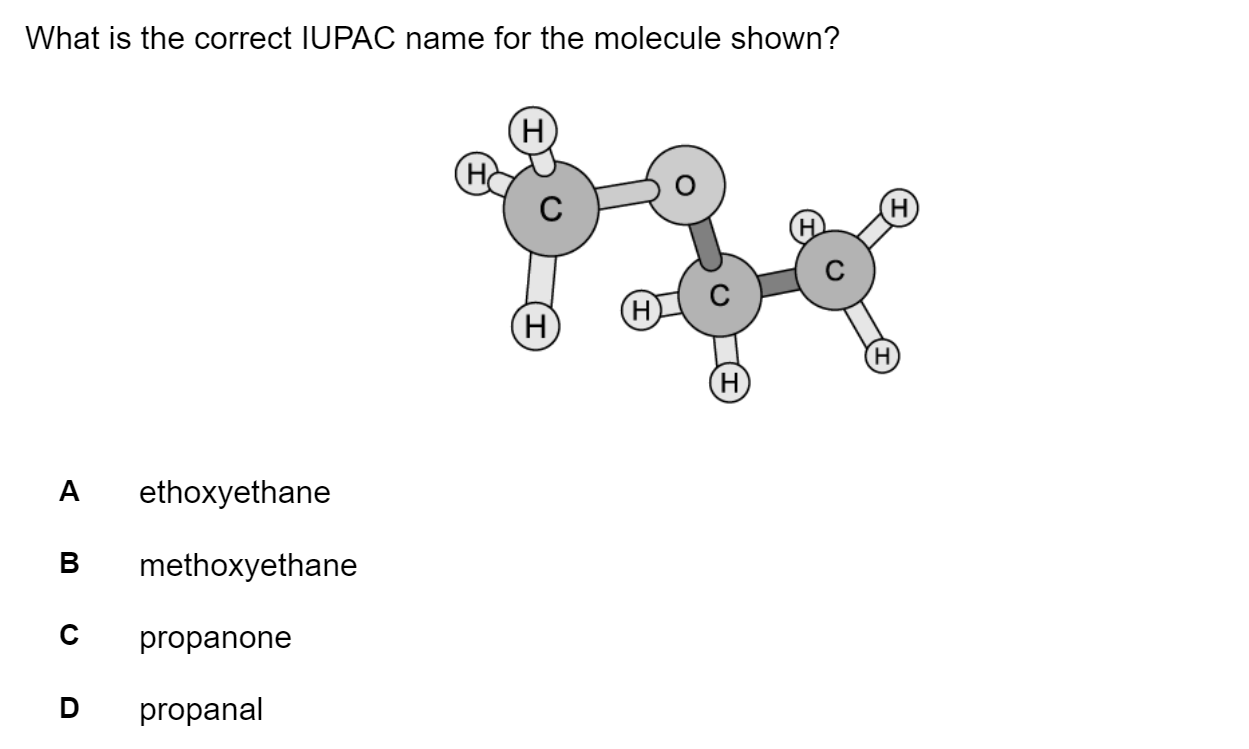
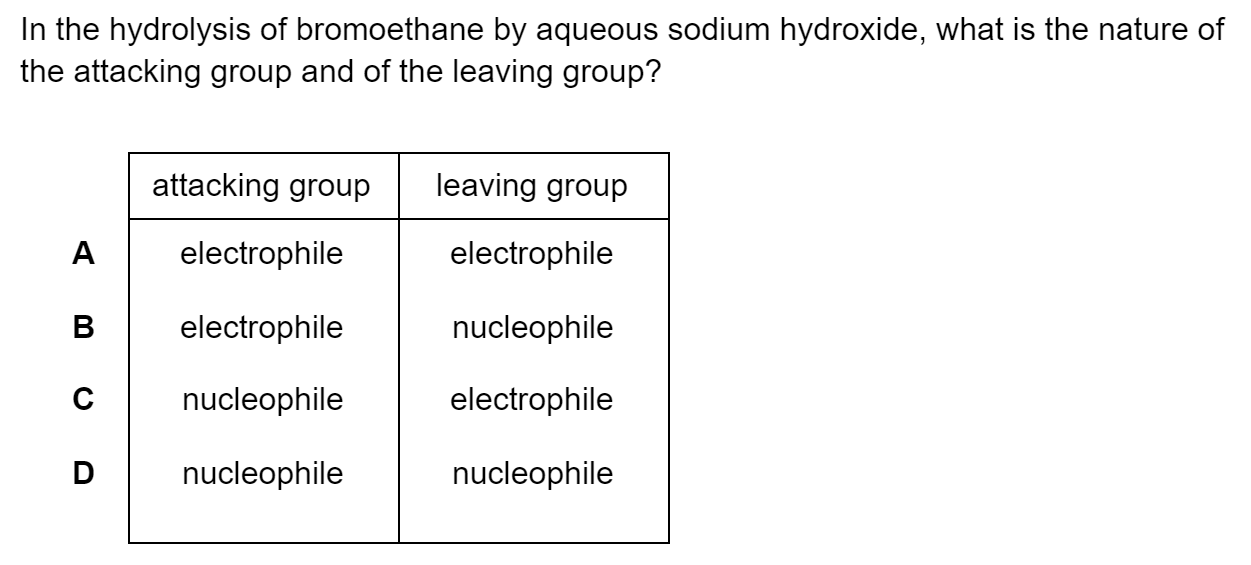
Which of the following statements describes first ionisation energy?
The electronegativity of four elements are given below
N = 3.0 H = 2.1 F = 4.0 P = 2.1
What is the correct order of polarity for the following compounds
PH3 < PF3 < NF3 < NH3
PH3 < PF3 < NH3 < NF3
NF3 < NH3 < PH3 < PF3
PH3 < NH3 < NF3 < PF3
Which of the species shown below does not have resonance structures?
C6H6
CO32-
C2H4
O3
What is the strongest type of intermolecular force exhibited in the amino acid molecule serine?

London dispersion forces
Permanent dipole permanent dipole forces
Hydrogen bonding
Covalent bonding
Which of the following molecules will have the highest boiling point?
CH3CH2CHO
CH3CH2CH2NH2
CH3CH2OCH3
CH3CH2CH2F
An experiment was carried out to determine the approximate value for the molar enthalpy change of neutralisation.
75 cm3 of 3.00 mol dm–3 hydrochloric acid was placed in a polystyrene beaker of negligible heat capacity. Its temperature was recorded, and then 75 cm3 of
3.00 mol dm−3 potassium hydroxide at the same temperature was quickly added, and the solution stirred.
The temperature rose by 14 °C. The resulting solution may be considered to have a specific heat capacity of 4.18 J g-1 K-1.
Which calculation below is correct?
J mol-1
J mol-1
J mol-1
J mol-1
The equations below show the formation of sulfur oxides from sulfur and oxygen.
S(s) + O2(g) → SO2(g) = –297 kJ mol–1
S(s) + 1½O2(g) →SO3(g) = –395 kJ mol–1
What is the enthalpy change of reaction,,of 2SO2(g) + O2(g) → 2SO3(g) in kJ mol–1?
(794 − 594)
(296 + 395)
(− 395 + 297)
(−790 + 594)
The reaction pathway for a reversible reaction is shown below:
Which statement is correct?
The activation energy of the reverse reaction is +90 kJ mol–1
The activation energy of the forward reaction is +20 kJ mol–1
The activation of the reverse reaction is +20 kJ mol–1
The enthalpy change of forwards reaction is - 70 kJ mol–1
The diagram shows the skeletal formula of cyclobutane.

The enthalpy change of formation of cyclobutane is +75.1 kJ mol–1, and the enthalpy change of atomisation of graphite is +712 kJ mol–1.
The bond enthalpy of C–H is 414 kJ mol–1 and of H–H is 436 kJ mol–1.
What is the average bond enthalpy of the C–C bond in cyclobutane?
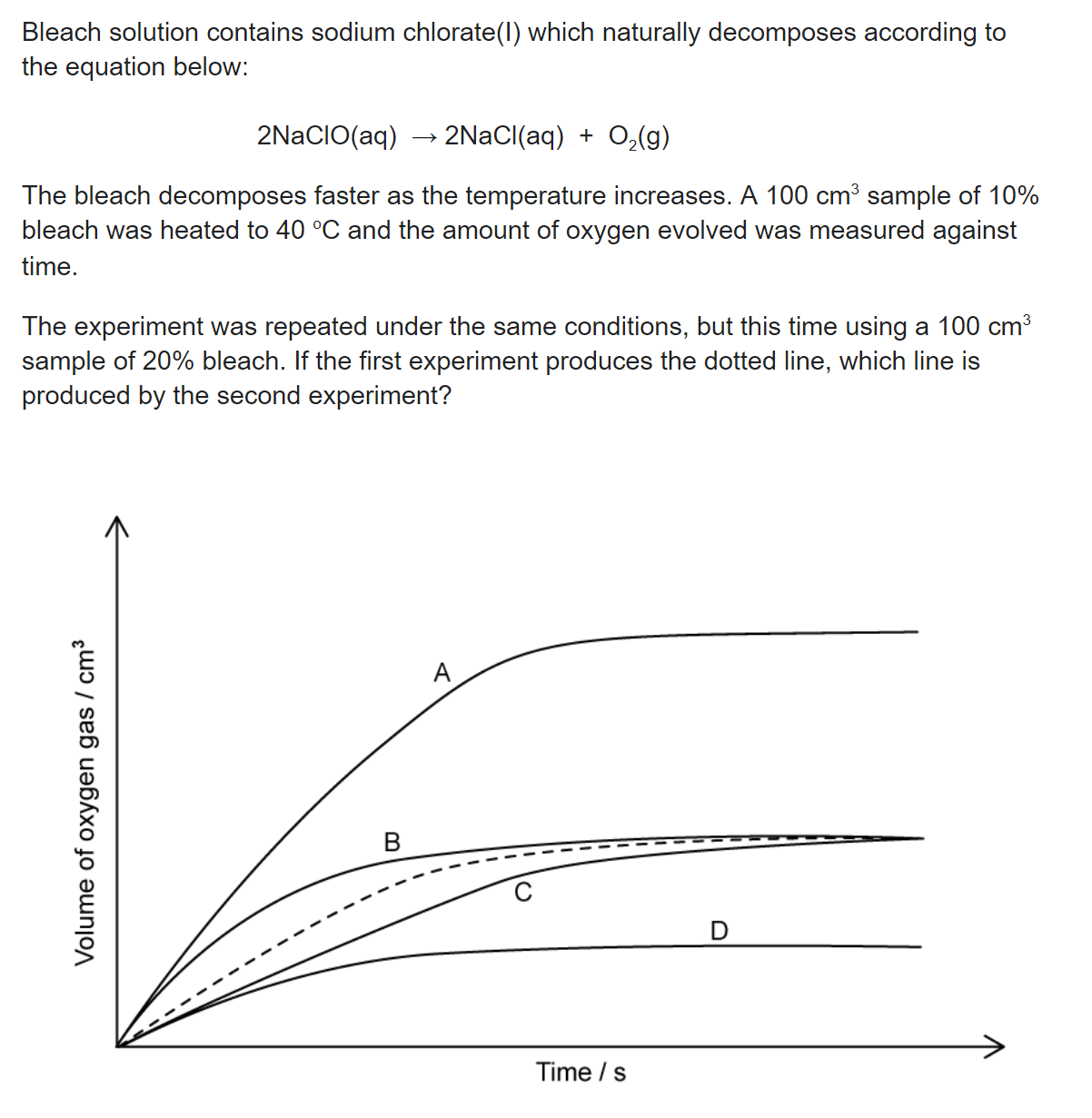
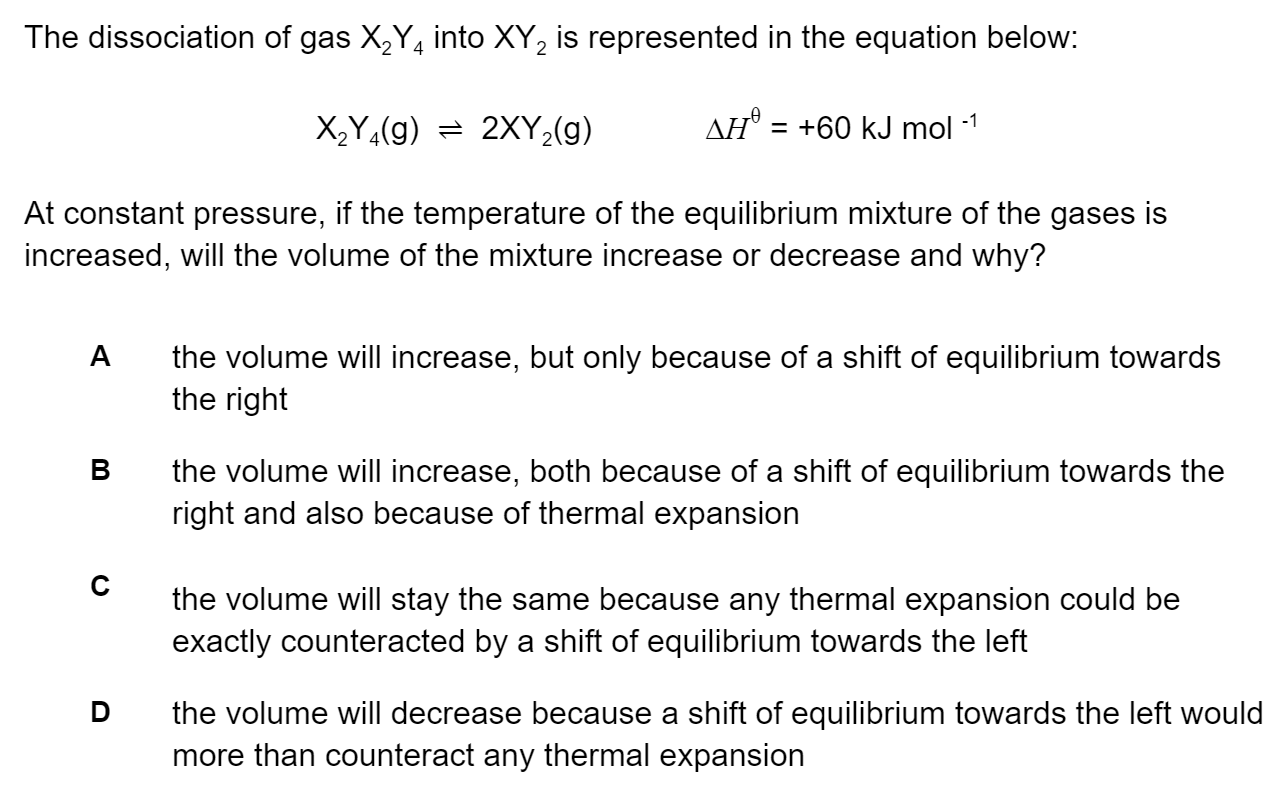
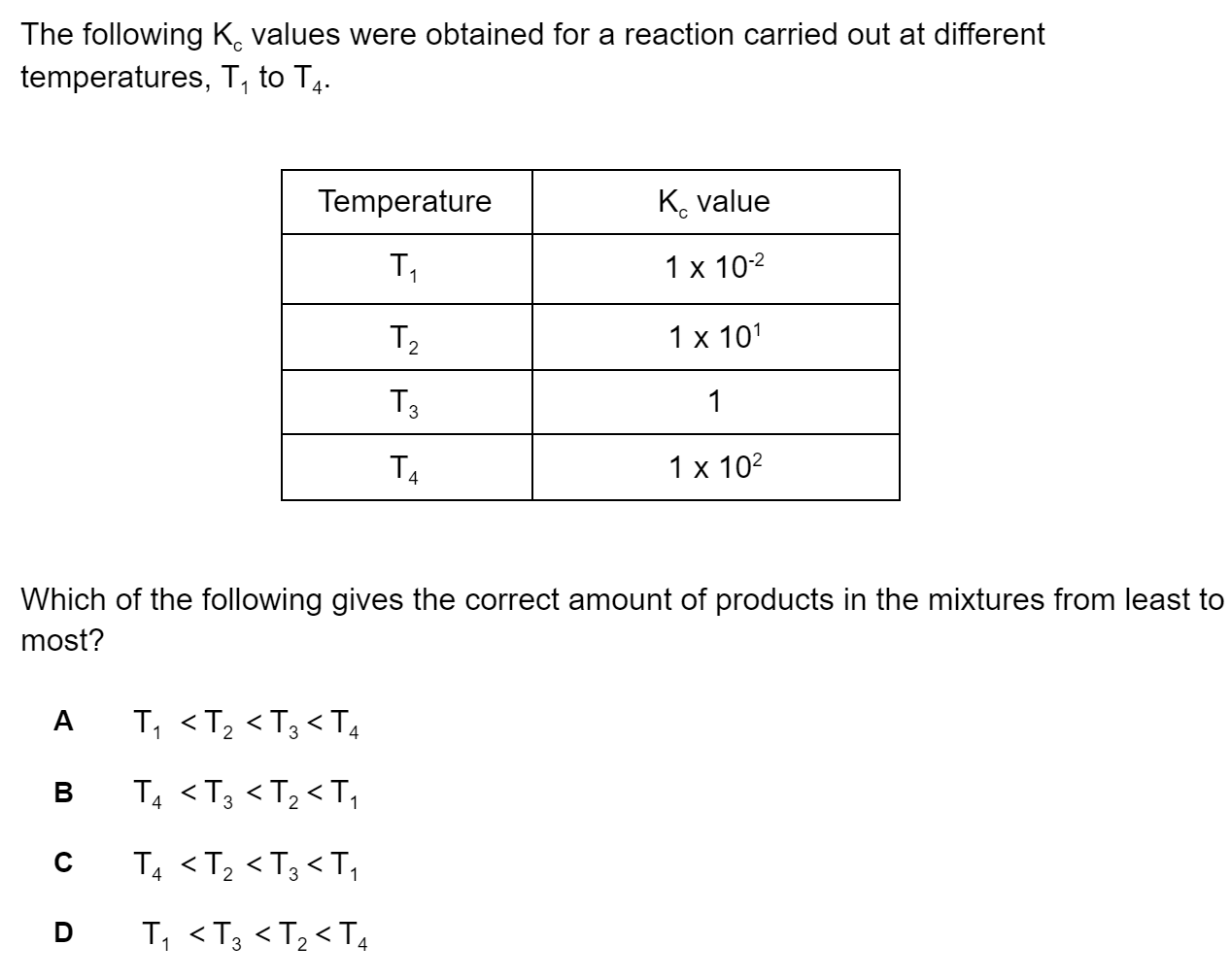
What role does each species play in the equilibrium below according to Brønsted-Lowry theory?
CH3COOH + HCl ⇌ CH3COOH2+ + Cl-
|
|
CH3COOH |
HCl |
CH3COOH2+ |
Cl- |
|
A |
acid |
base |
base |
acid |
|
B |
acid |
base |
acid |
base |
|
C |
base |
acid |
base |
acid |
|
D |
base |
acid |
acid |
base |
Four 1.0 M solutions of HCl, NH3, NaOH and CH3COOH have been mislabelled, but a student has a pH meter to test the pH of the solutions. Arrange the solutions in order of increasing pH:
HCl (aq) < NH3 (aq) < NaOH (aq) < CH3COOH (aq)
CH3COOH (aq) < HCl (aq) < NH3 (aq) < NaOH (aq)
HCl (aq) < CH3COOH (aq) < NH3 (aq) < NaOH (aq)
NaOH (aq) < NH3 (aq) < CH3COOH (aq) < HCl (aq)
The pH of clean rain water is around 5.5. Which substance is responsible for this?
Methane
Carbon dioxide
Nitrogen oxides
Sulfur dioxide