Question 1a
a)
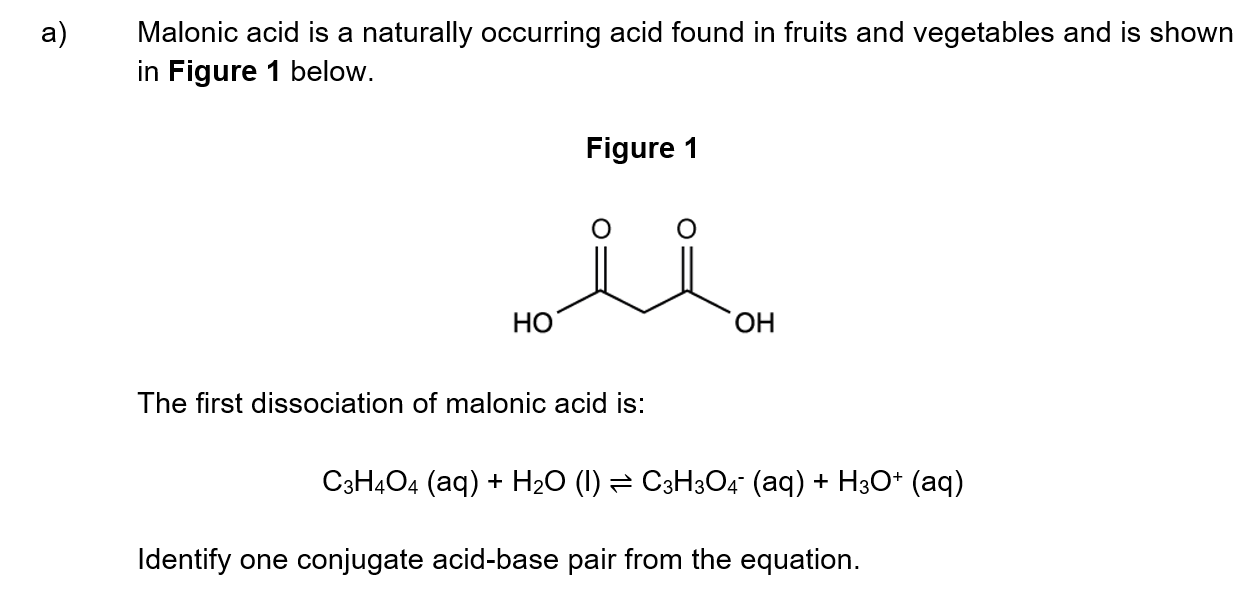
Nitroglycerin is an oily, colourless liquid and a high explosive, discovered by Alfred Nobel. The unbalanced equation for its explosive decomposition is given below.
........C3H5N3O9 (l) → .......N2 (g) + ......CO (g) + .......H2O (g) + .......O2 (g)
Deduce the coefficients required to balance the equation for this reaction and use the equation to suggest why nitroglycerin acts as a high explosive.
[2]
Question 1b
b)
Nitroglycerin is also used medicinally to treat angina attacks. It comes in the form of tablets, ointments, skin patches and nasal sprays. Nasal sprays vaporise the nitroglycerin, so it is quickly absorbed in the body.
A commercial 11.2 g nasal spray pump delivers a metered dose of exactly 400 micrograms of nitroglycerin. Determine the number of moles present in one dose and how many doses a spray pump can deliver.
[4]
Question 1c
c)
Suggest a reason why the actual number of doses delivered by the spray pump is less than you have calculated in (b).
[1]
Question 1d
d)
Describe the changes of state and the energy changes that take place when the spray pump is used.
[1]