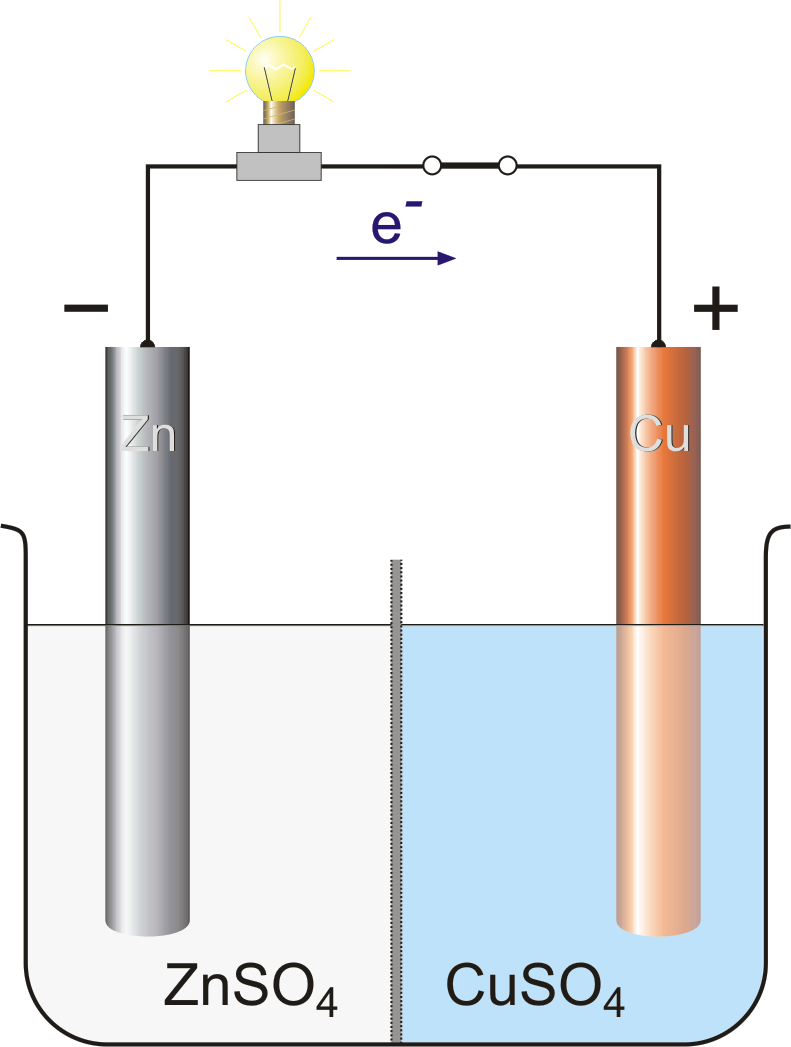
 Electrochemical cells is undoubtedly one of the most challenging topics in the course to get the head around. The principles of redox are straightforward - the loss and gain of electrons - but the concept is applied in different contexts in voltaic and electrochemical cells. Watch the video, read the revision cards and test yourself. If you really get to grips with understanding the differences between the cells it is no mean feat!
Electrochemical cells is undoubtedly one of the most challenging topics in the course to get the head around. The principles of redox are straightforward - the loss and gain of electrons - but the concept is applied in different contexts in voltaic and electrochemical cells. Watch the video, read the revision cards and test yourself. If you really get to grips with understanding the differences between the cells it is no mean feat!
Image E. Generalic, https://glossary.periodni.com/glossary.php?en=Daniell+cell
Ensure you are confident using the terms below and learn the asterisked* definitions
Electrolytic cell, Electrolysis, Voltaic cell, Electrode, Electrolyte, Spontaneous reaction, Non-spontaneous reaction, Potential difference, Half-cell, Cathode, Anode, Cell reaction, Cell notation, Salt bridge, Voltmeter
Which of the following apply to a voltaic cell?
1: The cell involves a spontaneous redox reaction.
2: Oxidation occurs at the cathode/the positive electrode.
3: The electrons flow from the negative electrode to the positive electrode.
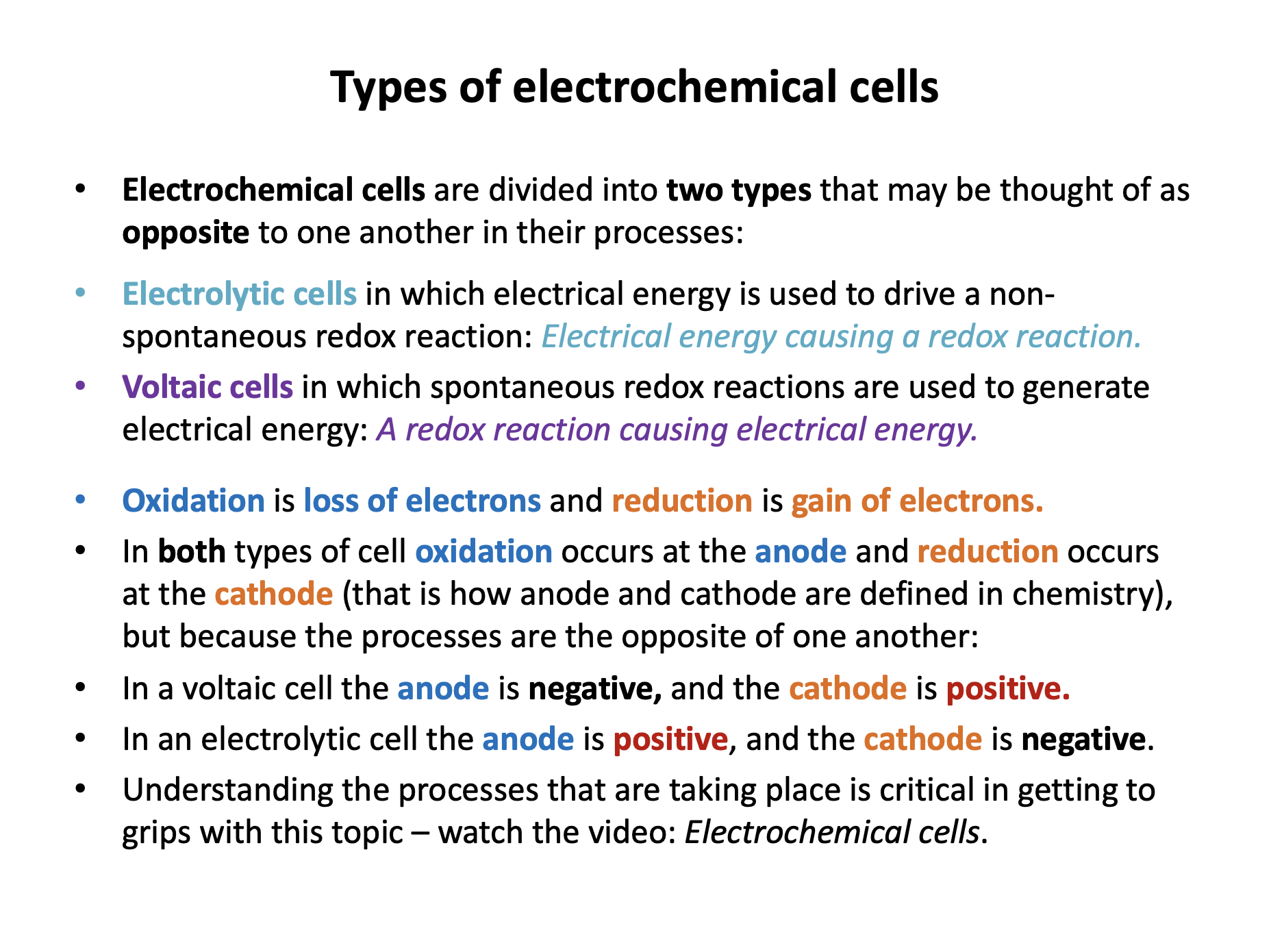
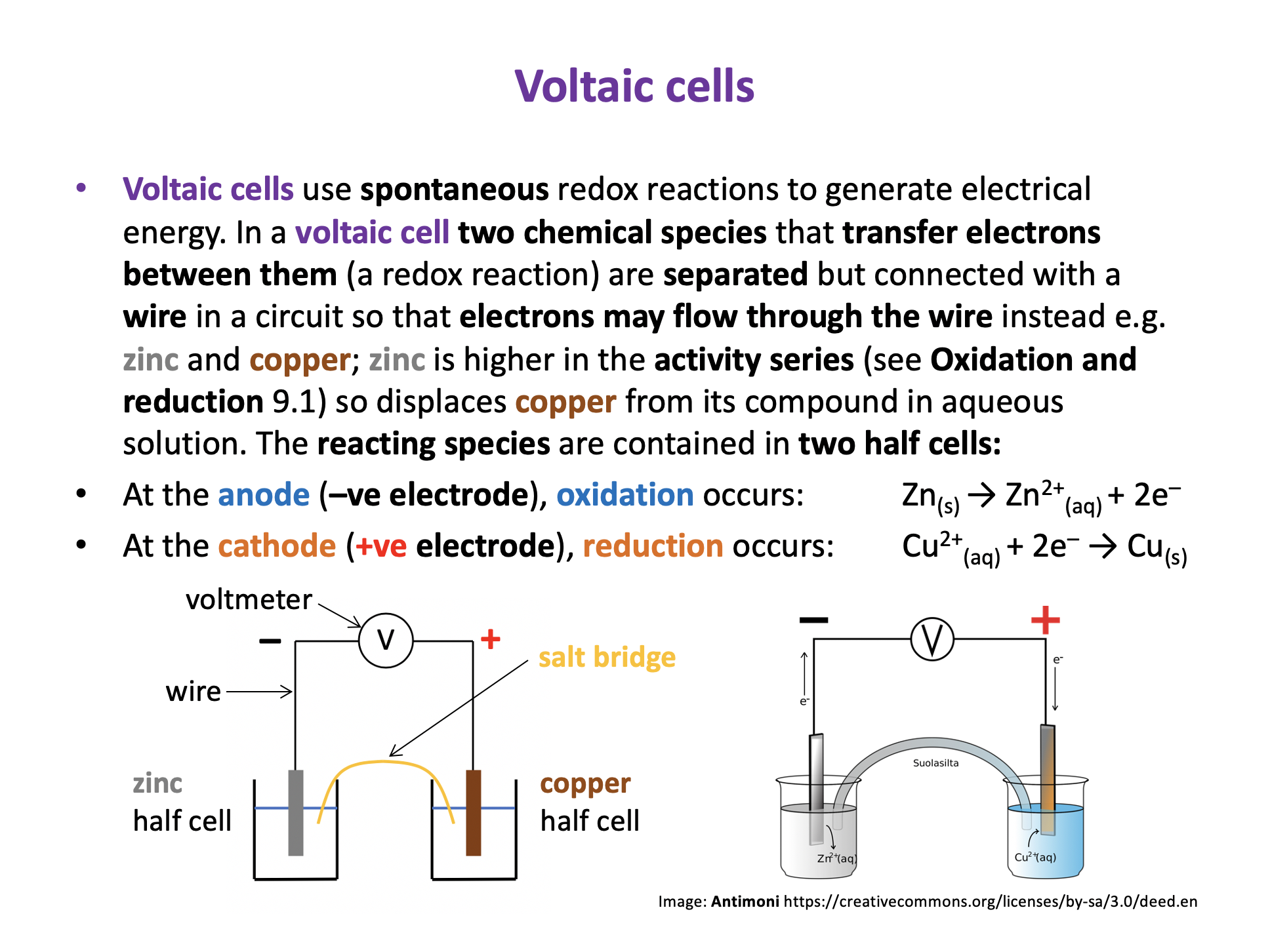
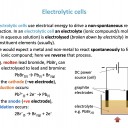
Voltaic cells use spontaneous redox reactions to generate electrical energy. Electrolytic cells use electrical energy to drive non-spontaneous redox reactions.
Thus statement 1 does apply to voltaic cells.
In both types of cell oxidation occurs at the anode and reduction occurs at the cathode. In a voltaic cell the anode is negative, and the cathode is positive. In an electrolytic cell the anode is positive, and the cathode is negative.
Thus statement 2 does not apply to voltaic cells (or to electrolytic cells).
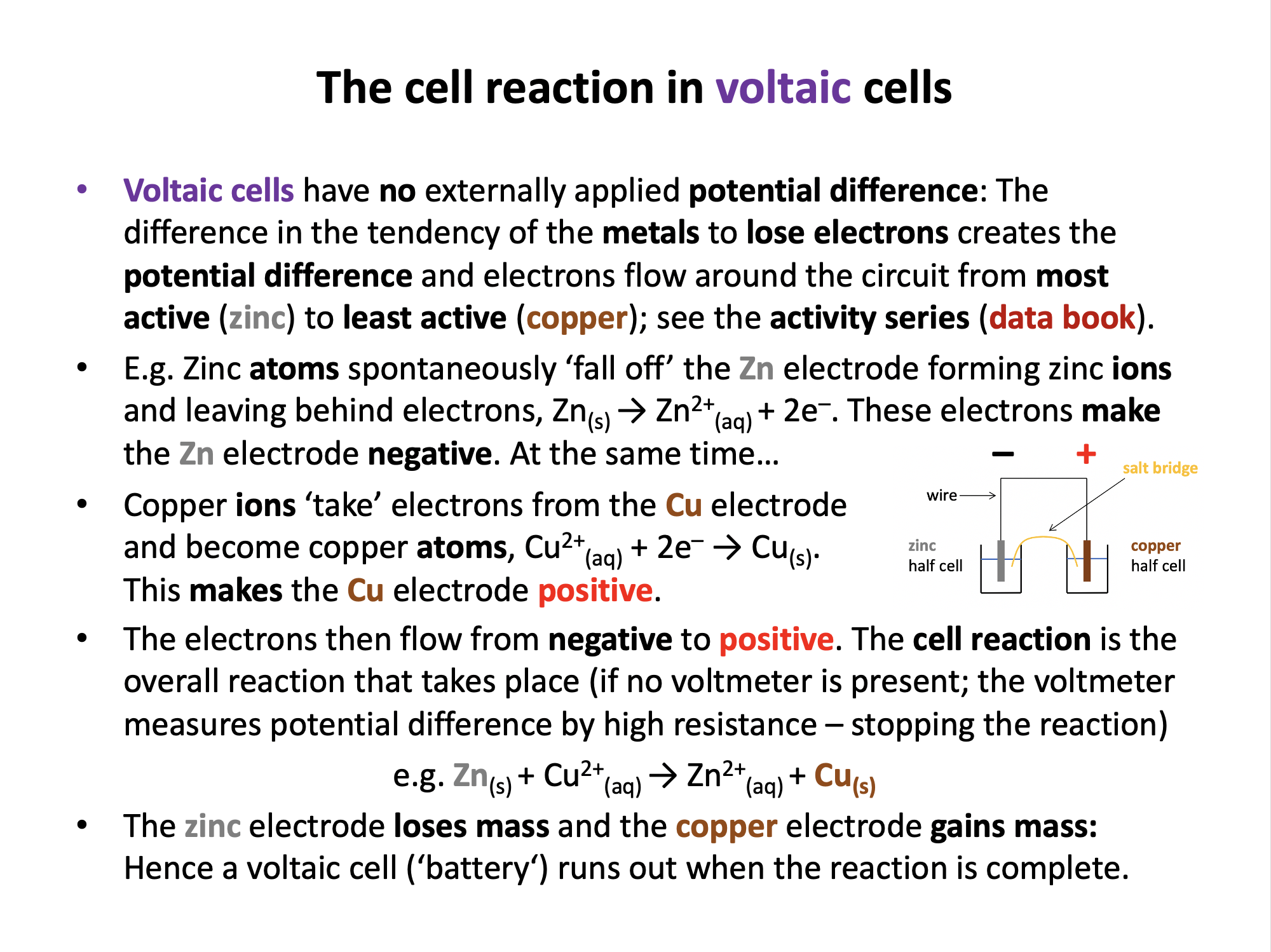
In a voltaic cell the redox reaction causes the electrodes to become charged and this generates electron flow from negative electrode (anode) to positive electrode (cathode).
Thus statement 3 does apply to voltaic cells.
Therefore 1 and 3 only is the correct answer.
Which of the following apply to an electrolytic cell?
1: The cell involves a spontaneous redox reaction.
2: Oxidation occurs at the anode/the positive electrode.
3: The electrolyte must be an electrical conductor in which ions can move.
Voltaic cells use spontaneous redox reactions to generate electrical energy. Electrolytic cells use electrical energy to drive non-spontaneous redox reactions.
Thus statement 1 does not apply to electrolytic cells.
In both types of cell oxidation occurs at the anode and reduction occurs at the cathode. In a voltaic cell the anode is negative, and the cathode is positive. In an electrolytic cell the anode is positive, and the cathode is negative.
Thus statement 2 does apply to electrolytic cells.
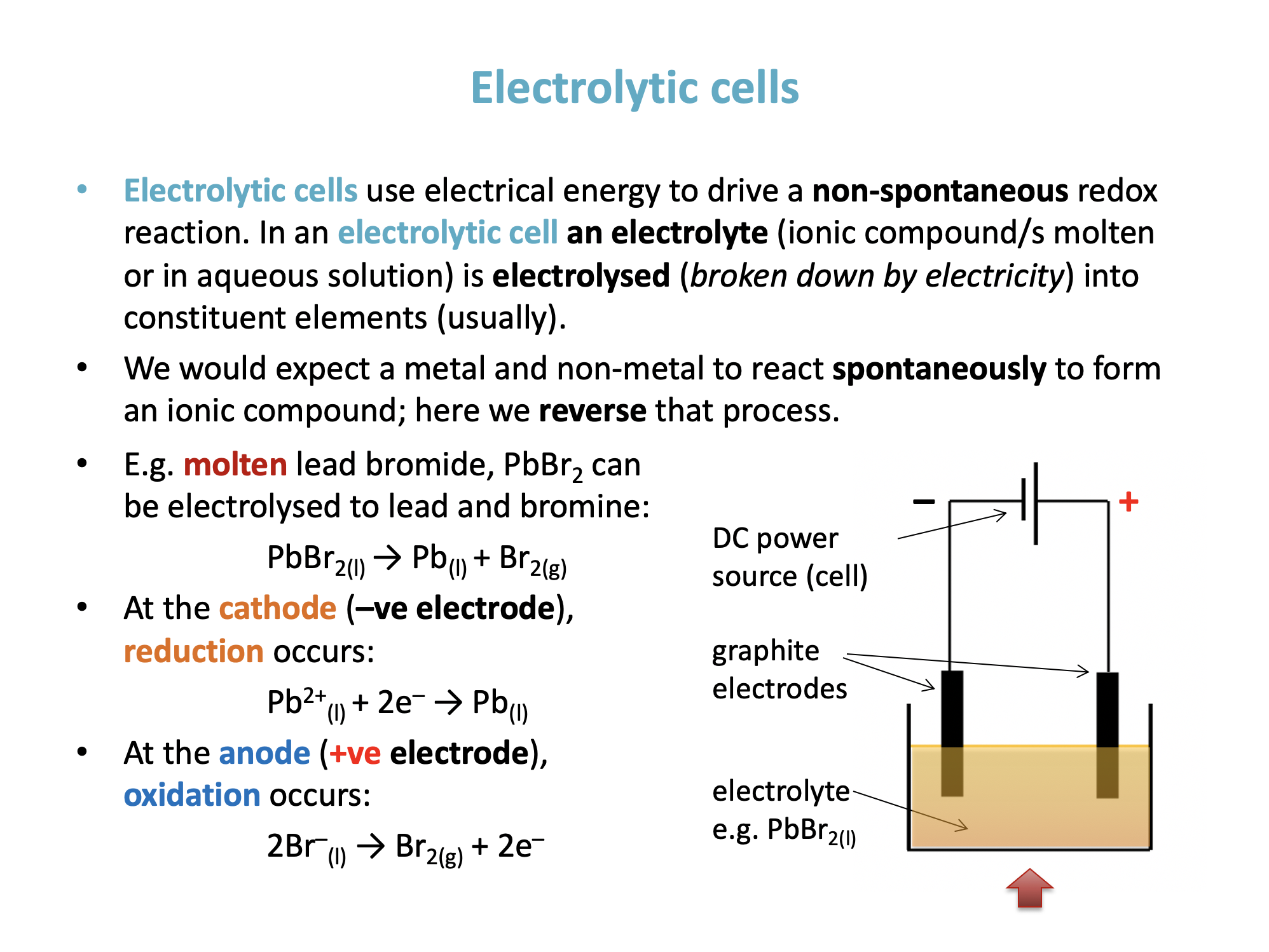
In an electrolytic cell the electrolyte is a molten ionic compound or an ionic compound in aqeous solution, both of which are electrical conductors in which ions can move.
Thus statement 3 does apply to electrolytic cells.
Therefore 2 and 3 only is the correct answer.
In the electrolysis of molten sodium bromide the sodium ion...
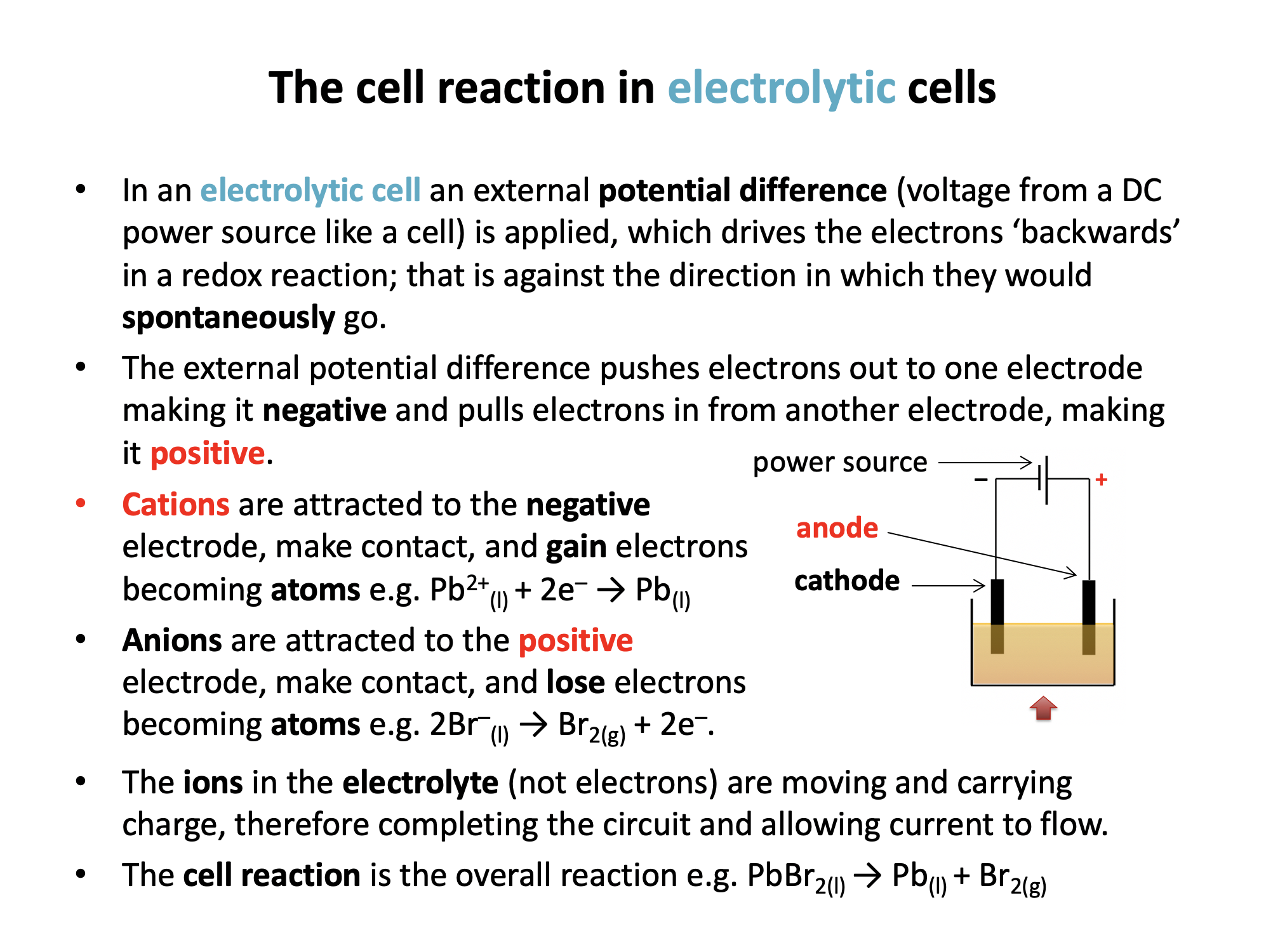
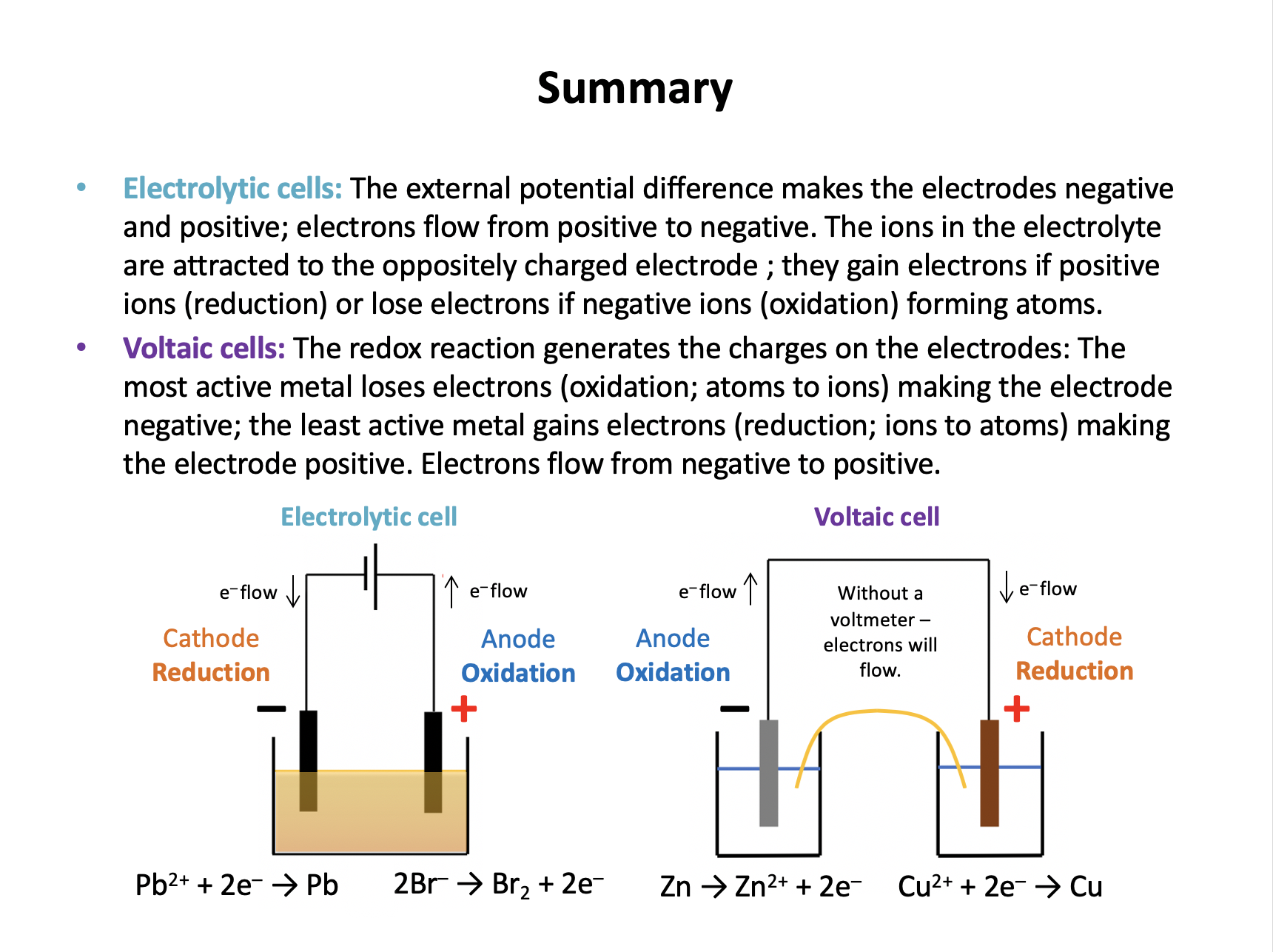
In an electrolytic cell the external potential difference pushes electrons out to one electrode making it negative and pulls electrons in from another electrode, making it positive. The ions in the electrolyte are then attracted to the oppositely charged electrode where they gain electrons (if positive ions) or lose electrons (if negative ions) to form atoms.
The sodium ion, being Na+ will go to the negative electrode and gain an electron which is reduction - RIG.
Thus goes to the negative electrode where it is reduced is the correct answer.
Which of the following apply to the electrolysis of lead bromide?
1: Lead ions go to the anode where they are oxidised.
2: Lead ions go to the cathode where they are reduced.
3: Bromide ions go to the anode where they are oxidised.
4: Bromide ions go to the cathode where they are reduced.
In an electrolytic cell the external potential difference pushes electrons out to one electrode making it negative and pulls electrons in from another electrode, making it positive. The ions in the electrolyte are then attracted to the oppositely charged electrode where they gain electrons (if positive ions) or lose electrons (if negative ions) to form atoms.
The lead ions, being Pb2+ will go to the negative electrode (cathode) and gain electrons which is reduction (RIG).
The bromide ions, being Br– will go to the positive electrode (anode) and lose electrons which is oxidation (OIL).
Thus 2 and 3 only is the correct answer.
What happens at the negative electrode in a voltaic cell and an electrolytic cell respectively?
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The most active metal loses electrons (oxidation; atoms to ions) causing the electrode to be negative, and the least active metal gains electrons (reduction; ions to atoms) causing the electrode to be positive.
So in a voltaic cell metal atoms will lose electrons (oxidation) at the negative electrode.
In an electrolytic cell the external potential difference pushes electrons out to one electrode making it negative and pulls electrons in from another electrode, making it positive. The ions in the electrolyte are then attracted to the oppositely charged electrode where they gain electrons (if positive ions) or lose electrons (if negative ions) to form atoms.
So in an electrolytic cell the positive ions will gain electrons (reduction) at the negative electrode.
Thus Oxidation; Reduction is the correct answer.
Which of the following statements about a voltaic cell comprising copper and iron half-cells are correct?
1: Electrons flow from the copper half-cell to the iron half-cell.
2: Oxidation takes place in the iron half-cell.
3: Negative ions travel across the salt bridge from the copper half-cell to the iron half-cell.
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The most active metal loses electrons (oxidation; atoms to ions) causing the electrode to be negative, and the least active metal gains electrons (reduction; ions to atoms) causing the electrode to be positive.
Iron is more active than copper (Activity series in the data book) so the iron half-cell will lose electrons (oxidation) and the copper half-cell will gain electrons (reduction); electrons will flow from the iron to the copper.
As electrons are flowing from iron to copper, the charge will be equalised by negative ions flowing across the salt bridge from the copper to the iron half-cell.
Thus 2 and 3 only is the correct answer.
Using the information given in the reactions of fictional metal sulfates below,
X(s) + YSO4(aq) → XSO4(aq) + Y(s)
Y(s) + ZSO4(aq) → no reaction
which of the following statements is correct?
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The most active metal loses electrons (oxidation; atoms to ions) causing the electrode to be negative, and the least active metal gains electrons (reduction; ions to atoms) causing the electrode to be positive. Electrons flow from negative to positive.
The equations tell us that X is more active than Y (X displaces Y) and that Z is more active than Y (Y cannot displace Z).
In a voltaic cell of Y and Z, Z loses electrons (oxidation) and Y gains electrons (reduction). Electrons flow from Z to Y.
In a voltaic cell of X and Y, X loses electrons (oxidation) and Y gains electrons (reduction). Electrons flow from X to Y.
Thus the correct answer is A voltaic cell with half-cells of metals Y and Z will result in electrons flowing from Z to Y
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The most active metal loses electrons (oxidation; atoms to ions) causing the electrode to be negative, and the least active metal gains electrons (reduction; ions to atoms) causing the electrode to be positive. Electrons flow from negative to positive.
Magnesium is more active than zinc (activity series in data book).
Therefore magnesium loses electrons (oxidation) and zinc gains electrons (reduction) and the electrons flow from magnesium to zinc.
Thus the correct answer is Reduction takes place in the zinc half-cell
This question refers to voltaic cells, but the only information needed is that magnesium is more active than copper (activity series in data book).
The strongest reducing agent (reduction is gain of electrons) is the species most likely to lose electrons and give them to something else, thereby reducing it. Magnesium atoms have the greatest tendency to lose electrons as they are the most active (copper is less likely to lose electrons, and magnesium and copper ions, having already lost two electrons are unlikely to lose any more).
Thus the correct answer is Mg
Which shows the correct cell notation for a voltaic cell consisting of magnesium and iron(II) half-cells?
Magnesium is more active than iron (activity series in data book). Magnesium will lose electrons (oxidation) and iron will gain electrons (reduction). Electrons will flow from magnesium to iron.
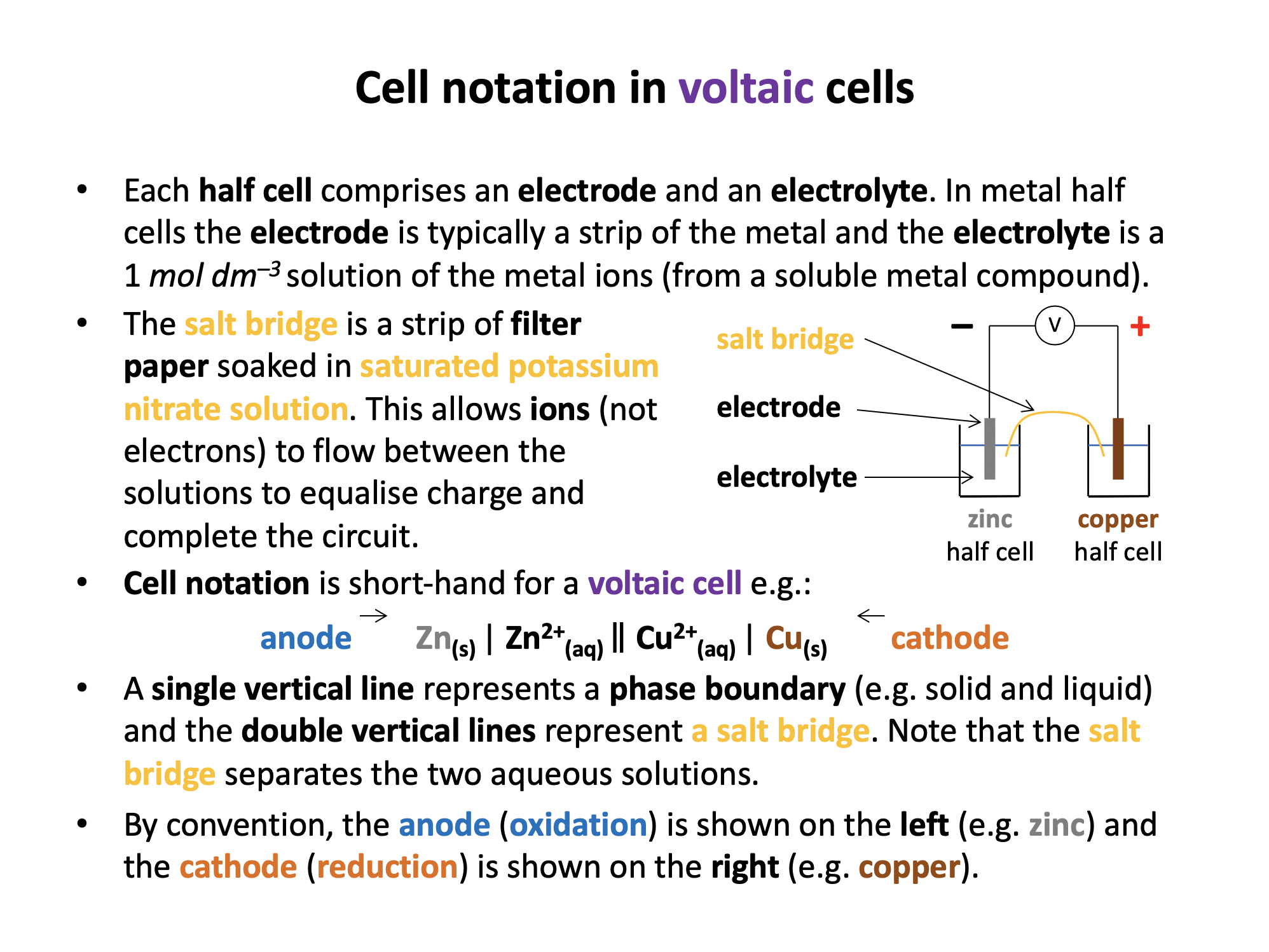
By convention, the anode (oxidation) is shown on the left (magnesium) and the cathode (reduction) is shown on the right (iron). A single vertical line represents a phase boundary (e.g. solid and liquid) and the double vertical lines represent a salt bridge. The salt bridge should separate the two aqueous solutions.
Thus Mg(s) | Mg2+(aq) || Fe2+(aq) | Fe(s) is the correct answer.
Paper 1
Core (SL&HL): Redox core (SL and HL) paper 1 questions
AHL (HL only): Redox AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Redox core (SL & HL) paper 2 questions
AHL (HL only): Redox AHL (HL only) paper 2 questions
How much of Electrochemical cells I have you understood?











 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn