 It is important to understand that redox is about electron counting. If you understand our oxidation state model, then the rest of the Redox topic will be more accessible and less confusing. We are always asking ourselves 'Which atoms have the valance electrons?'. In ionic compounds that's easy to answer (see Ionic bonding 4.1), since in our model electrons transfer. In covalent compounds we assign the electrons in bonds to the atoms with the highest electronegativity. This is the basis of our oxidation state model - keep that in mind; despite all the stuff to learn, the principle of oxidation and reduction is a simple one.
It is important to understand that redox is about electron counting. If you understand our oxidation state model, then the rest of the Redox topic will be more accessible and less confusing. We are always asking ourselves 'Which atoms have the valance electrons?'. In ionic compounds that's easy to answer (see Ionic bonding 4.1), since in our model electrons transfer. In covalent compounds we assign the electrons in bonds to the atoms with the highest electronegativity. This is the basis of our oxidation state model - keep that in mind; despite all the stuff to learn, the principle of oxidation and reduction is a simple one.
Ensure you are confident using the terms below and learn the asterisked* definitions
Redox, Oxidation*, Reduction*, Oxidising agent*, Reducing agent*, Oxidation state, Oxidation number, Half-equation, Activity series, The Winkler method, Biological oxygen demand
Which of the following describes oxidation?
1: The gain of oxygen.
2: The loss of hydrogen.
3: The gain of electrons.
'1 and 2 only' is the correct answer.
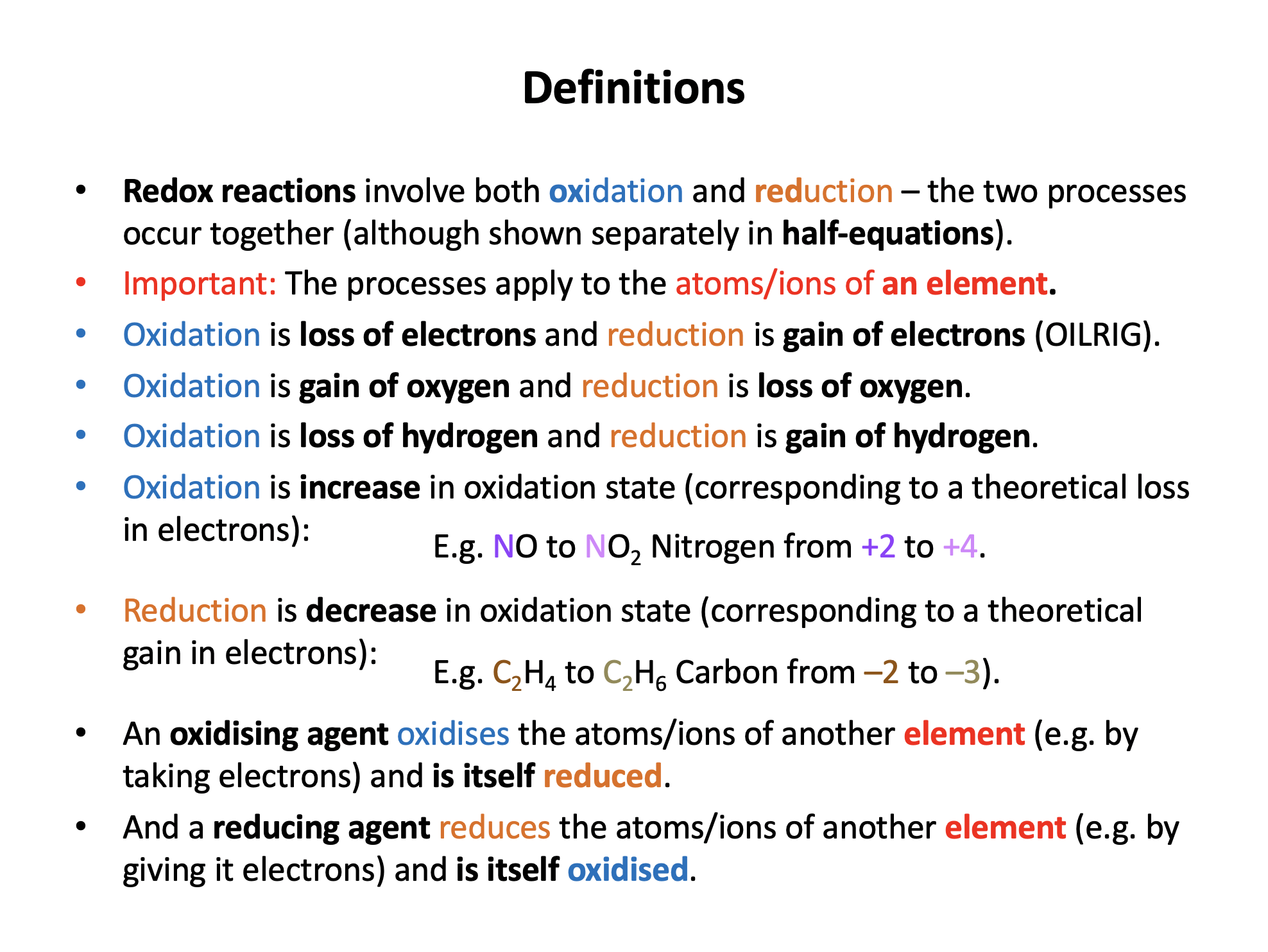
Oxidation can be defined in terms of an element gaining oxygen or losing hydrogen (both useful in organic chemistry), or may be defined as loss of electrons (OILRIG) or an increase in oxidation state.
Which of the following describes reduction?
1: The loss of oxygen or gain of hydrogen.
2: A decrease in oxidation state.
3: The gain of electrons.
Reduction can be defined in all of these ways. In terms of an element losing oxygen or gaining hydrogen (both useful in organic chemistry), or gaining electrons (OILRIG) or a decrease in oxidation state.
Thus 1, 2 and 3 is the correct answer.
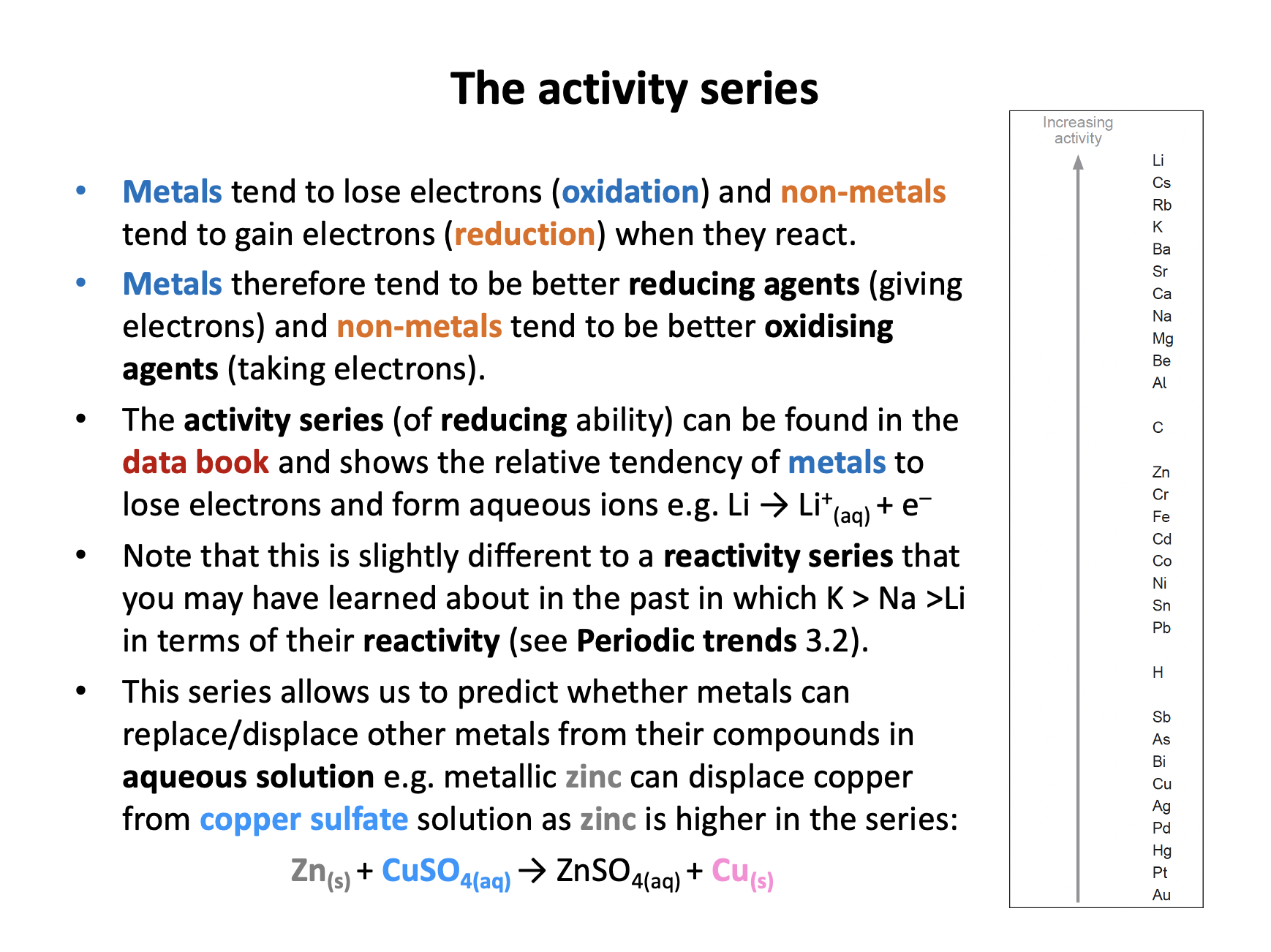
Which of the following reactions could theoretically occur as written, given sufficient activation energy?
1: Mg + Zn(NO3)2 → Mg(NO3)2 + Zn
2: Zn + MgSO4 → ZnSO4 + Mg
3: CuSO4 + Zn → ZnSO4 + Cu
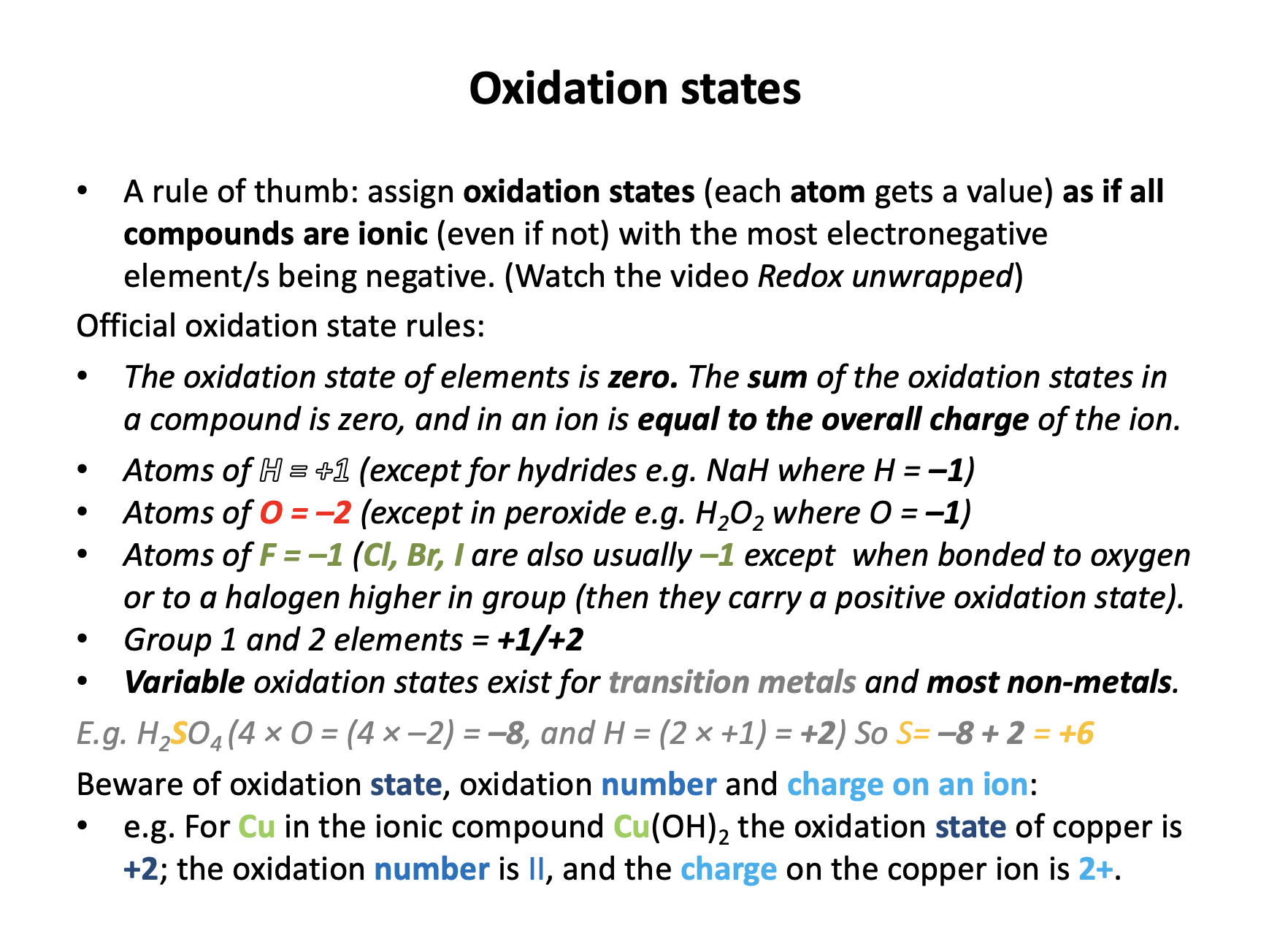
What is the oxidation state of chromium in potassium dichromate K2Cr2O7?
+6 is the correct answer, since each oxygen atom has an oxidation state of −2 and each potassium +1.
(7 × −2) + (2 × +1) = −12 and the compound is neutral overall (0), so the two chromium atoms carry +12, thus +6 for each atom. The oxidation state of chromium is +6.
Note that 6+ is a charge, oxidation states must be shown with the + or − sign before the number. When oxidation states are assigned, it is as if everything is ionic, but this is not the case.
What is the oxidation state of chlorine in the chlorate ion ClO3−?
+5 is the correct answer, since oxygen is the most electronegative element and will therefore carry the negative oxidation state; each oxygen atom has an oxidation state of −2.
(3 × −2) = −6 and the compound has a 1− charge (notice for the charge the number comes first) which means the ion has an overall oxidation state of −1, so the chlorine atom has an oxidation state of +5 (−6 + +5 = −1).
What is the oxidation number of iron in Fe2O3?
'III' is the correct answer; oxidation numbers are represented by Roman numerals (e.g. iron (III) oxide or copper (II) oxide) and oxidation states by numbers with the charge in front.
In Fe2O3 each oxygen has an oxidation state of −2; 3 × −2 = −6 and the compound is neutral overall (0), so the two irons carry +6, thus +3 for each. The oxidation state of iron is +3. And this compound is ionic, so the charge on the iron ion is 3+.
What is the name of the compound MnO2?
'Manganese (IV) oxide' is the correct answer.
In MnO2 each oxygen atom has an oxidation state of −2; 2 × −2 = −4 and the compound is neutral overall (0), so the manganese atom has an oxidation state of +4, this is represented by the oxidation number of IV in the name.
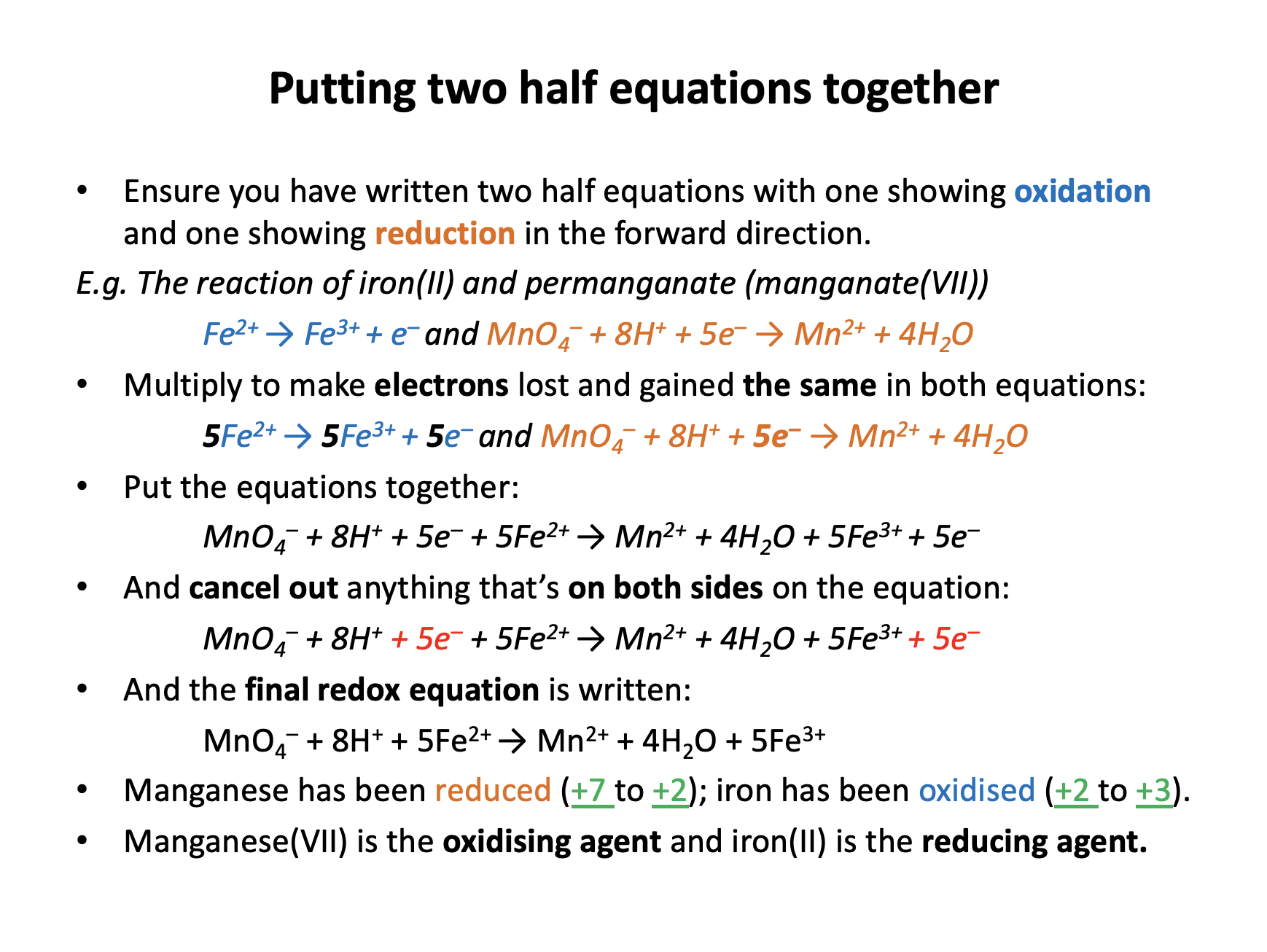
Iron (II) ions react with hydrogen peroxide in acidic solution according to the following equation:
2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
Which chemical species is the reducing agent in this reaction?
A reducing agent reduces another element and therefore is itself oxidised (OILRIG: if it gives electrons away to another element, it has lost electrons).
Oxidation state changes are needed:
Iron goes from +2 to +3 (Fe2+ to Fe3+).
Oxygen goes from −1 (always in a peroxide) to −2 (H2O2 to H2O).
Hydrogen is +1 throughout.
Thus iron is oxidised and oxygen is reduced. The Fe2+ ions are the reducing agent; 'giving an electron' to oxygen.
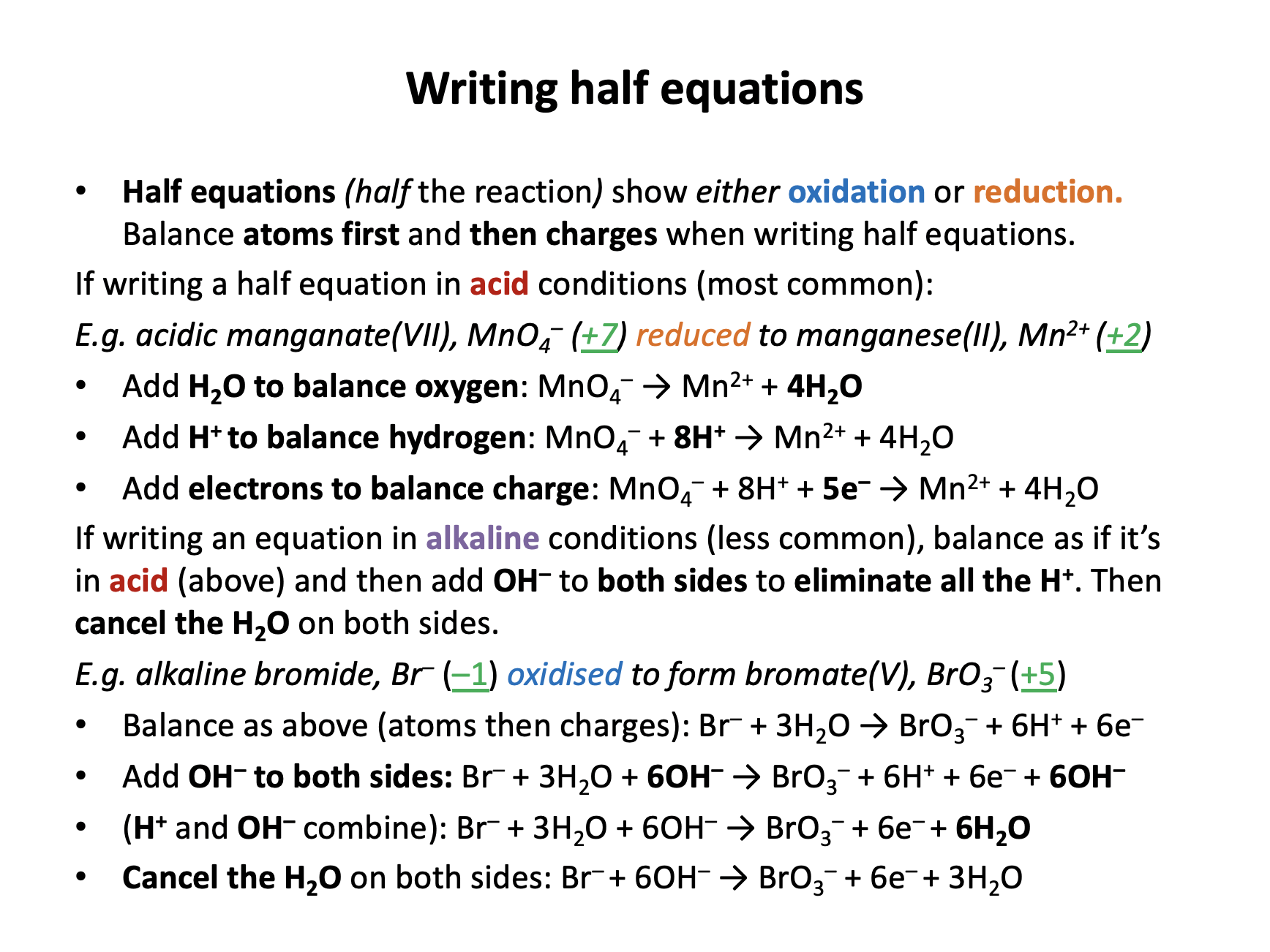
Balance the following redox equation to give the lowest whole number ratio:
____OH− + ____SO32− + ____Fe3+ → ____SO42− + ____H2O + ____Fe2+
How many moles of iron (III) ions are required?
Always balance the electrons first by calculating changes in oxidation states:
Oxygen remains as −2 throughout.
Hydrogen remains as +1 throughout.
Sulfur changes from +4 to +6 (loss of 2 electrons).
Iron changes from +3 to +2 (gain of 1 electron).
To balance electrons lost and gained, two iron ions are needed:
____OH− + ____SO32− + 2Fe3+ → ____SO42− + ____H2O + 2Fe2+
Now atoms of other elements can be balanced:
2OH− + SO32− + 2Fe3+ → SO42− + H2O + 2Fe2+
So the correct answer is '2'.
Balance the following redox equation to give the lowest whole number ratio:
____H+ + ____BiO3− + ____Mn2+ → ____Bi3+ + ____H2O + ____MnO4−
How many moles of H+ ions are required?
Always balance the electrons first by calculating changes in oxidation states:
Oxygen remains as −2 throughout.
Hydrogen remains as +1 throughout.
Bismuth (Bi) changes from +5 to +3 (gain of 2 electrons).
Manganese (Mn) changes from +2 to +7 (loss of 5 electrons).
To balance electrons lost and gained (10 electrons), five 'bismuths' are needed and two 'manganeses':
____H+ + 5BiO3− + 2Mn2+ → 5Bi3+ + ____H2O + 2MnO4−
Now atoms of other elements can be balanced:
Oxygens:
____H+ + 5BiO3− + 2Mn2+ → 5Bi3+ + 7H2O + 2MnO4−
Then hydrogens:
14H+ + 5BiO3− + 2Mn2+ → 5Bi3+ + 7H2O + 2MnO4−
So the correct answer is '14'.
The Winkler method is carried out to find the dissolved oxygen content of a sample of cold river water.
250cm3 of the water is taken and treated with manganese (II) chloride, sodium iodide, sodium hydroxide and finally sulphuric acid, to yield iodine. The iodine is titrated against thiosulphate ions.
On average 4.20 × 10−5 moles of thiosulphate ions are used in the redox titration for a 25.0 cm3 aliquot of the 'water solution'.
How many moles of dissolved oxygen are there in a 25.0 cm3 sample of the warm water?
The moles of dissolved oxygen in the aliquot is always one quarter of the moles of thiosulphate used in the titration:
4.20 × 10−5 / 4 = 1.05 × 10−5 moles
This, incidently, equates to 13.4 mg dm−3 which is the same as parts per million (in fact, mg per 1000g (1 dm3) of solution). A value of 13.4 ppm suggests that the river water is not likely to be polluted.
Working backwards through the Winkler method:
2S2O32-(aq) + I2 S4O62-(aq) + 2I-(aq)
2 moles of thiosulphate reacts with 1 mole of iodine (I2).
2Mn3+(aq) + 2I-(aq) I2 + 2Mn2+(aq)
1 mole of iodine is liberated by 2 moles of Mn3+ ions.
Mn(OH)3(s) + 3H+(aq) Mn3+(aq) + 3H2O
2 moles of Mn3+ ions are produced by 2 moles of manganese (III) hydroxide (1:1 ratio).
4Mn(OH)2 + O2 + 2H2O 4Mn(OH)3
2 moles of manganese (III) hydroxide is produced from ½ mole (ratio is 4:1) of dissolved oxygen.
So overall 4 moles of thiosulphate ions equates to 1 mole of dissolved oxygen.
Paper 1
Core (SL&HL): Redox core (SL and HL) paper 1 questions
AHL (HL only): Redox AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Redox core (SL & HL) paper 2 questions
AHL (HL only): Redox AHL (HL only) paper 2 questions
How much of Oxidation and reduction have you understood?



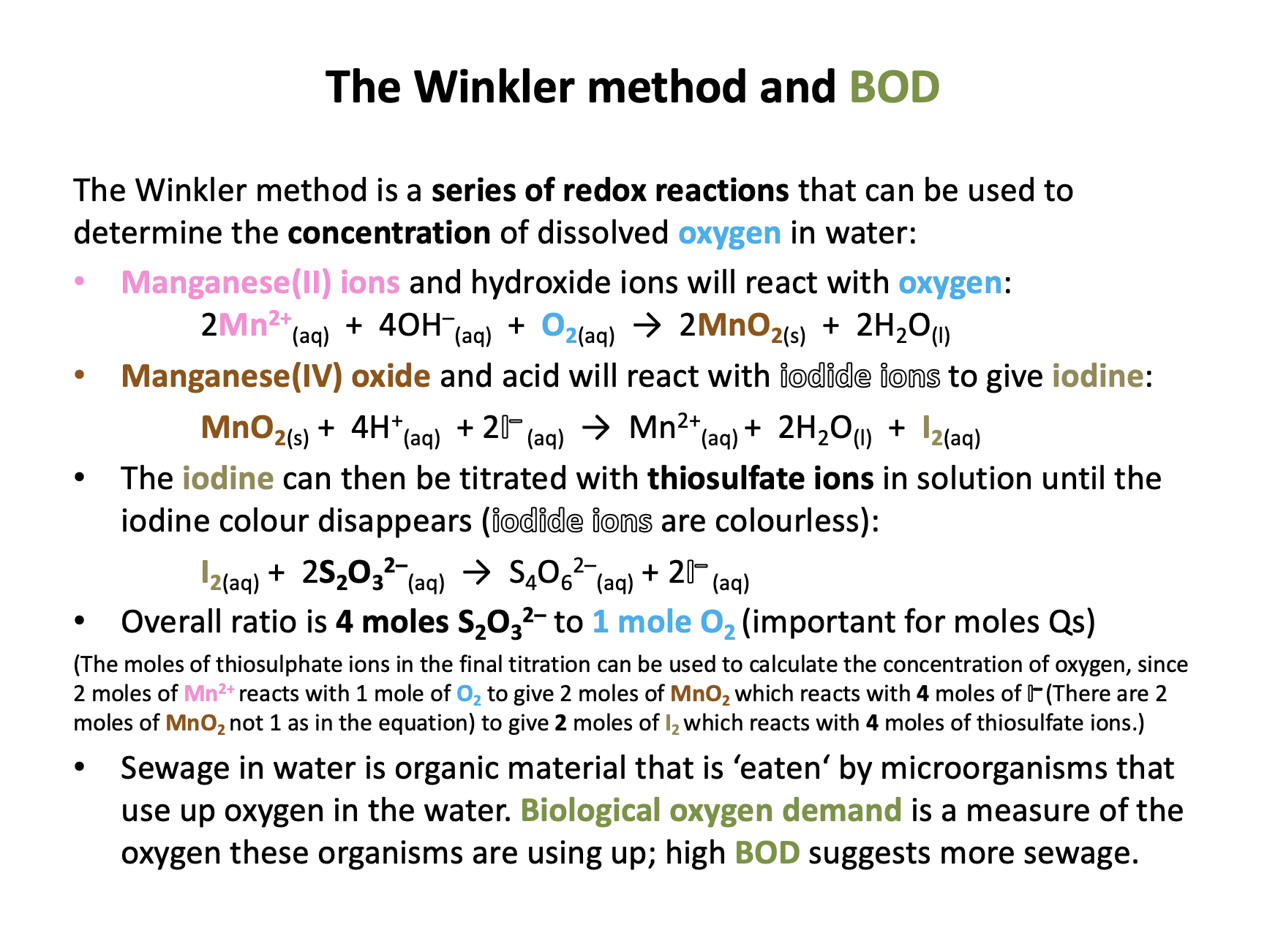








 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn