 It is absolutely critical that understanding of the topics oxidation and reduction 9.1 and electrochemical cells 9.2 is thorough before beginning on revision of this, more advanced, topic. It is so easy to get lost in this topic, and there is a lot to deal with. However, a really sound grasp of the earlier topics; knowing exactly how voltaic and electrolytic cells operate and where oxidation and reduction occurs will make this much easier, as we can always return to first principles and ask ourselves, Where are the electrons moving?
It is absolutely critical that understanding of the topics oxidation and reduction 9.1 and electrochemical cells 9.2 is thorough before beginning on revision of this, more advanced, topic. It is so easy to get lost in this topic, and there is a lot to deal with. However, a really sound grasp of the earlier topics; knowing exactly how voltaic and electrolytic cells operate and where oxidation and reduction occurs will make this much easier, as we can always return to first principles and ask ourselves, Where are the electrons moving?
Ensure you are confident using the terms below and learn the asterisked* definitions
Electrochemical series, Standard electrode potential*, Cell potential, Standard hydrogen electrode (SHE)*, Faraday constant, Electroplating, Absolute charge
Which of the following statements about a voltaic cell consisting of the zinc and iron half-cells below are correct?
Zn2+(aq) + 2e– ⇌ Zn(s) Eo = –0.76 V
Fe3+(aq) + e– ⇌ Fe2+(aq) Eo = +0.77 V
1: Electrons flow from the iron half-cell to the zinc half-cell.
2: The cell potential Eocell = +1.53 V
3: Negative ions travel across the salt bridge from the iron half-cell to the zinc half-cell.
In a voltaic cell the redox reaction generates the charges on the electrodes and causes the current to flow. The half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) causing the electrode to be negative, and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction) causing the electrode to be positive.
Zinc half-cell is more negative than the iron half-cell (Electrochemical series in the data book) so the zinc half-cell will lose electrons (oxidation) and the iron half-cell will gain electrons (reduction); electrons will flow from the zinc to iron.
Eocell = Eocathode – Eoanode = +0.77 – –0.76 = +1.53 V
As electrons are flowing from zinc to iron, the charge will be equalised by negative ions flowing across the salt bridge from the iron to the zinc half-cell.
Thus 2 and 3 only is the correct answer.
What is the overall cell reaction and cell potential for a voltaic cell consisting of the nickel and copper half-cells below?
Ni2+(aq) + 2e– ⇌ Ni(s) Eo = –0.26 V
Cu2+(aq) + 2e– ⇌ Cu(s) Eo = +0.34 V
In a voltaic cell the half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction).
Nickel half-cell is more negative than the copper half-cell (Electrochemical series in the data book) so the nickel half-cell will lose electrons (oxidation) and the copper half-cell will gain electrons (reduction):
Ni(s) → Ni2+(aq) + 2e– and Cu2+(aq) + 2e– → Cu(s)
The number of electrons lost and gained is the same so the half-equations combined:
Cu2+(aq) + Ni(s) ⇌ Ni2+(aq) + Cu(s)
Eocell = Eocathode – Eoanode = +0.34 – –0.26 = +0.60 V
Thus Cu2+(aq) + Ni(s) ⇌ Ni2+(aq) + Cu(s) Eocell = +0.60 V is the correct answer.
What is the overall cell reaction and cell potential for a voltaic cell consisting of the nickel and aluminium half-cells below?
Ni2+(aq) + 2e– ⇌ Ni(s) Eo = –0.26 V
Al3+(aq) + 3e– ⇌ Al(s) Eo = –1.66 V
In a voltaic cell the half-cell highest in the electrochemical series (most negative) loses electrons (oxidation) and the half-cell lowest in the electrochemical series (most positive) gains electrons (reduction).
Aluminium half-cell is more negative than the nickel half-cell (Electrochemical series in the data book) so the aluminium half-cell will lose electrons (oxidation) and the nickel half-cell will gain electrons (reduction):
Al(s) → Al3+(aq) + 3e– and Ni2+(aq) + 2e– → Ni(s)
The number of electrons lost and gained in each half-cell must be equalised when the half-equations combine (Al × 2, Ni × 3):
3Ni2+(aq) + 2Al(s) ⇌ 2Al3+(aq) + 3Ni(s)
Eocell = Eocathode – Eoanode = –0.26 – –1.66 = +1.40 V
Note that the Eo values are independent of the multiplication to equal out the number of electrons.
Thus 3Ni2+(aq) + 2Al(s) ⇌ 2Al3+(aq) + 3Ni(s) Eocell = +1.40V is the correct answer.
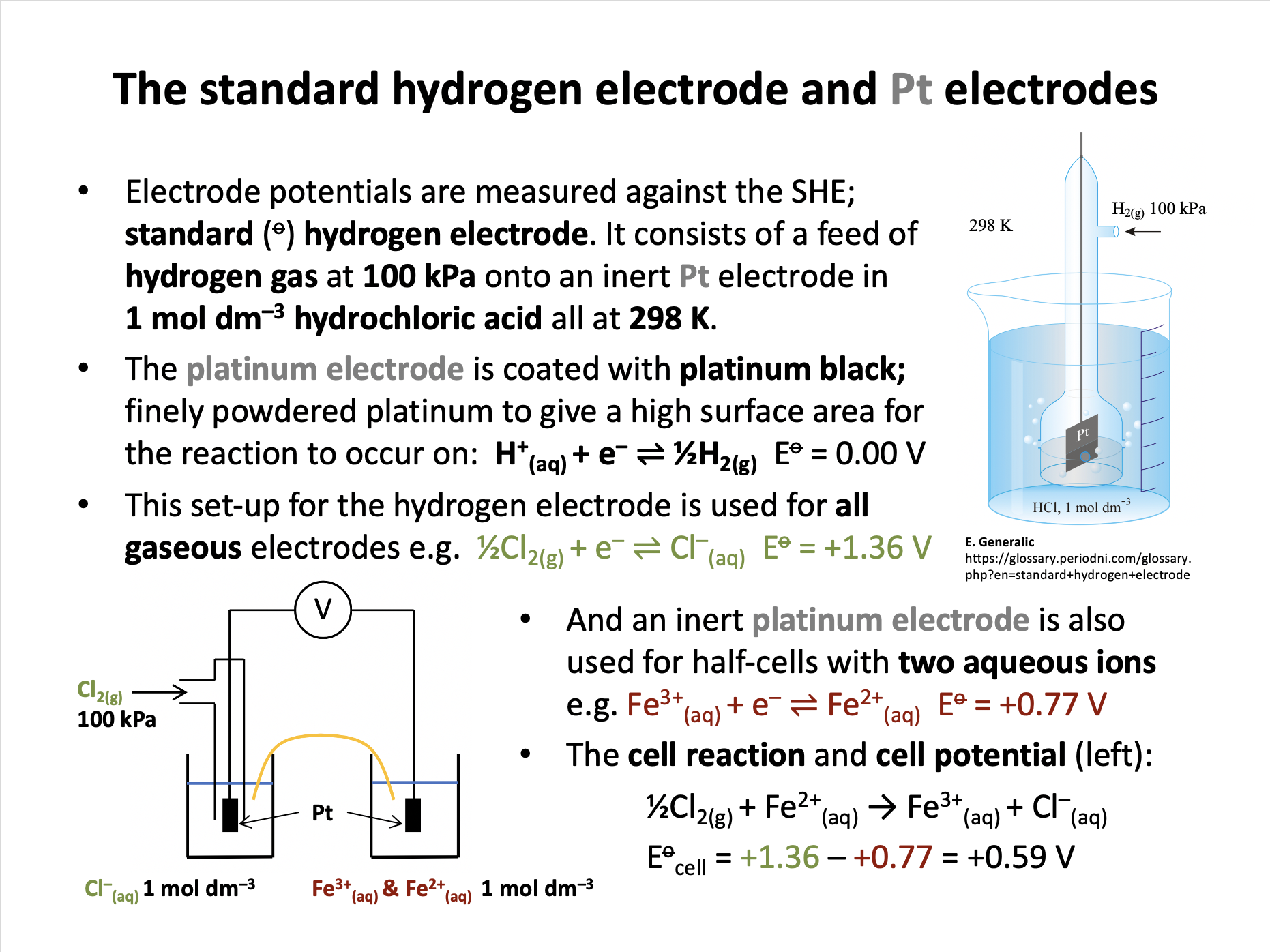
Which of the following statements about the standard hydrogen electrode (SHE) are correct?
1: The electrode is made of inert platinum.
2: Hydrogen gas is fed onto the electrode at 100kPa.
3: The electrode is immersed in hydrochloric acid (1 mol dm−3) at 298K.
Electrode potentials are measured against the SHE; standard hydrogen electrode.
The SHE consists of a feed of hydrogen gas at 100 kPa onto an inert Pt electrode in 1 mol dm–3 hydrochloric acid all at 298 K.
The platinum electrode is coated with platinum black; finely powdered platinum to give a high surface area for the reaction to occur on: H+(aq) + e– ⇌ ½H2(g) Eo = 0.00 V
Thus 1, 2 and 3 is the correct answer.
Which of the following half-cells does not require a platinum electrode?
An inert platinum electrode is needed for all gaseous electrodes (e.g. chlorine and hydrogen) and for half-cells involving only aqueous ions (e.g. Cu2+(aq) + e– ⇌ Cu+(aq)).
The copper half-cell Cu2+(aq) + 2e– ⇌ Cu(s) requires a copper electrode and is therefore the correct answer.
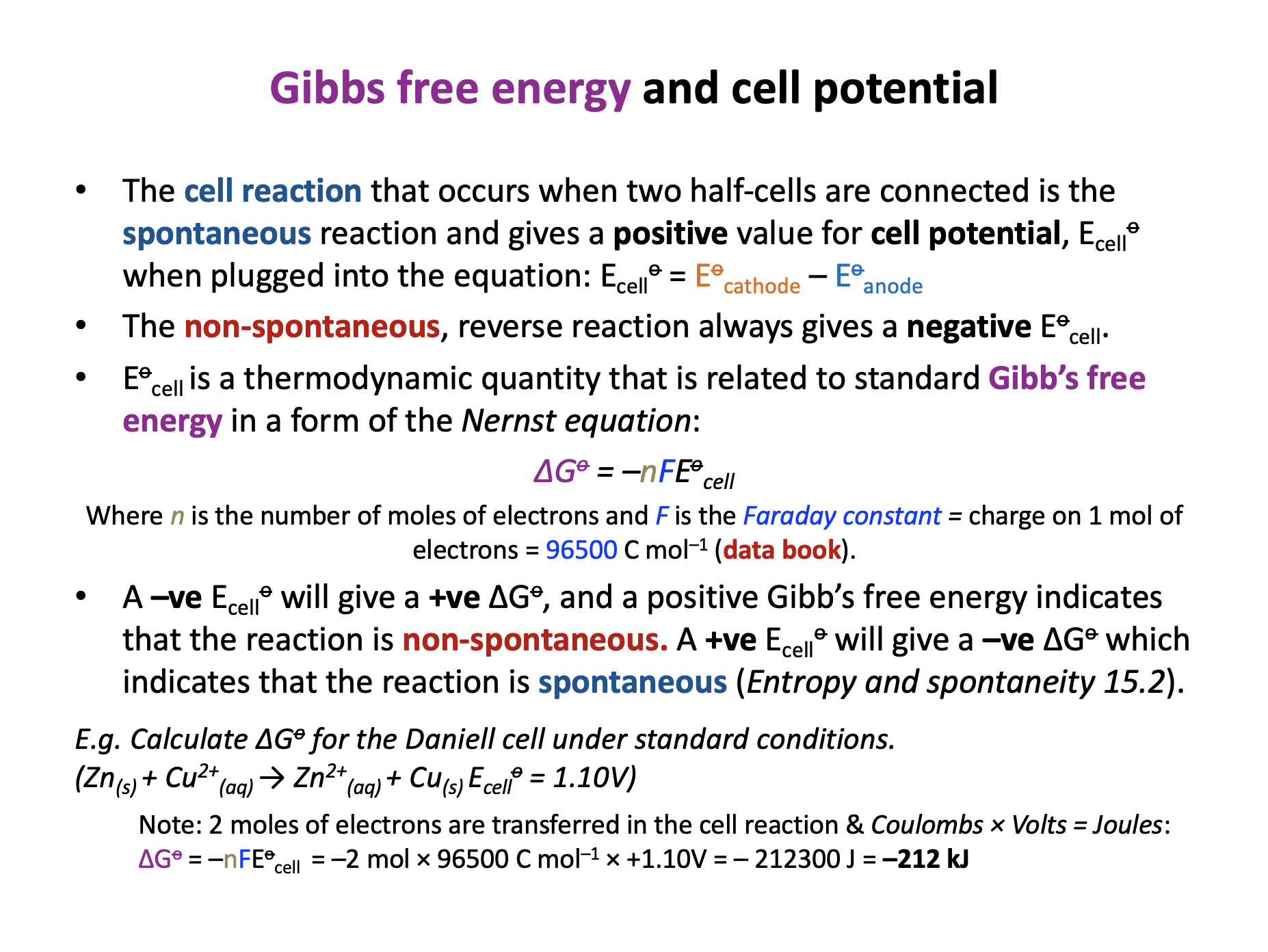
Calculator question: What is the value of ΔGo under standard conditions when calculated for the cell reaction in a voltaic cell consisting of the two half-cells below (in kJ to 3 sig figs)?
(using ΔGo= –nFEocell in data book)
Fe2+(aq) + 2e– ⇌ Fe(s) Eo = –0.45 V
Cu2+(aq) + 2e– ⇌ Cu(s) Eo = +0.34 V
Iron(II) half-cell is more negative than the copper half-cell (Electrochemical series in the data book) so the iron half-cell will lose electrons (oxidation) and the copper half-cell will gain electrons (reduction):
Fe(s) → Fe2+(aq) + 2e– and Cu2+(aq) + 2e– → Cu(s)
The number of electrons lost and gained is the same (that is two electrons) so the half-equations combined:
Cu2+(aq) + Fe(s) ⇌ Fe2+(aq) + Cu(s)
Eocell = Eocathode – Eoanode = +0.34 – –0.45 = +0.79 V
ΔGo = –nFEocell = – (2 mol × 96500 C mol–1 × +0.79 V) = – 152470 J = –152 kJ (3 sig figs)
Incorrect answers
–76.2 uses 1 mol of electrons instead of 2
+152 uses –0.79 V instead of +0.79 V (or emits to use the –ve sign in the equation)
+76.2 uses 1 mol of electrons instead of 2 and uses –0.79 V instead of +0.79 V (or emits to use the –ve sign in the equation)
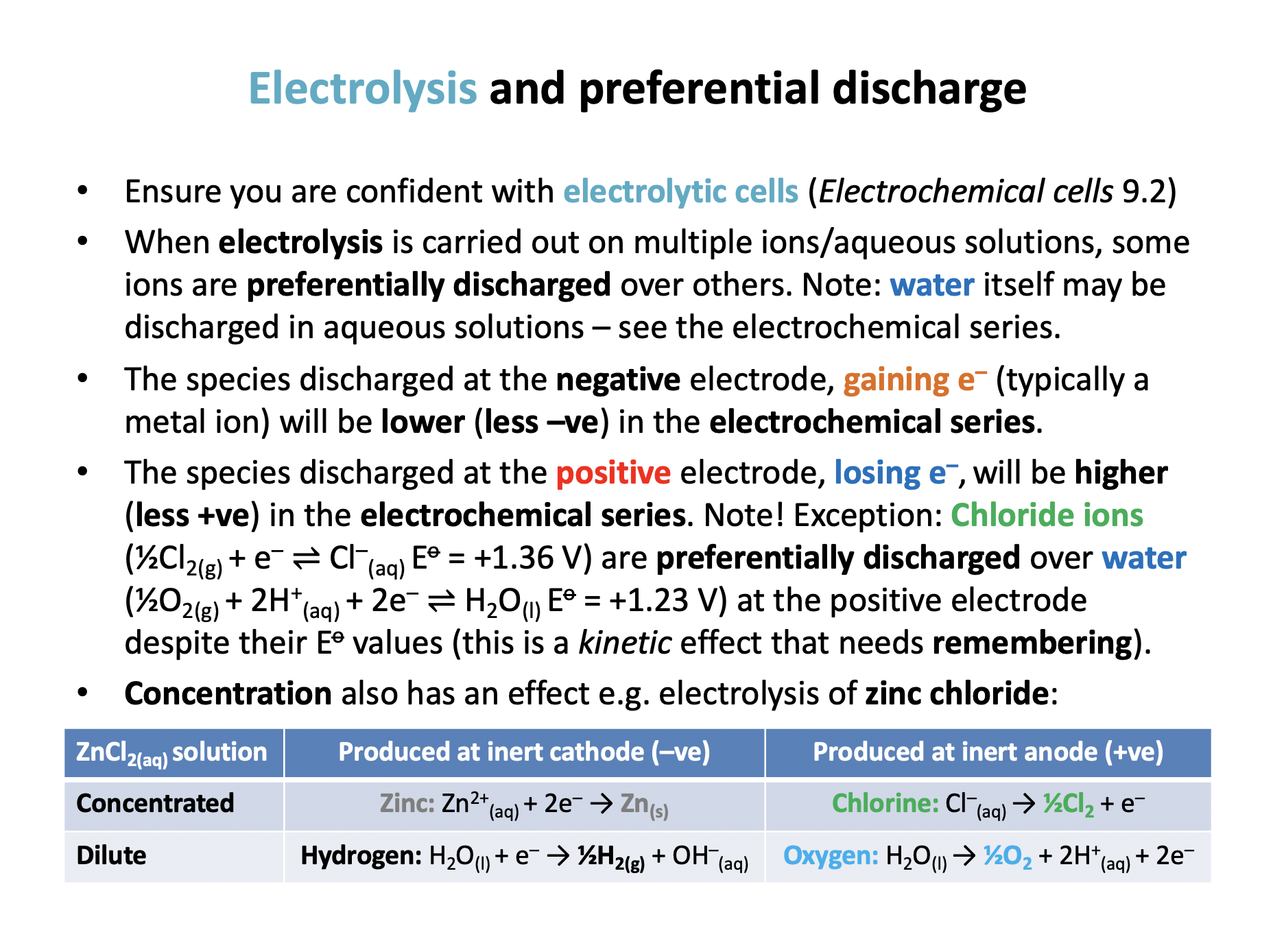
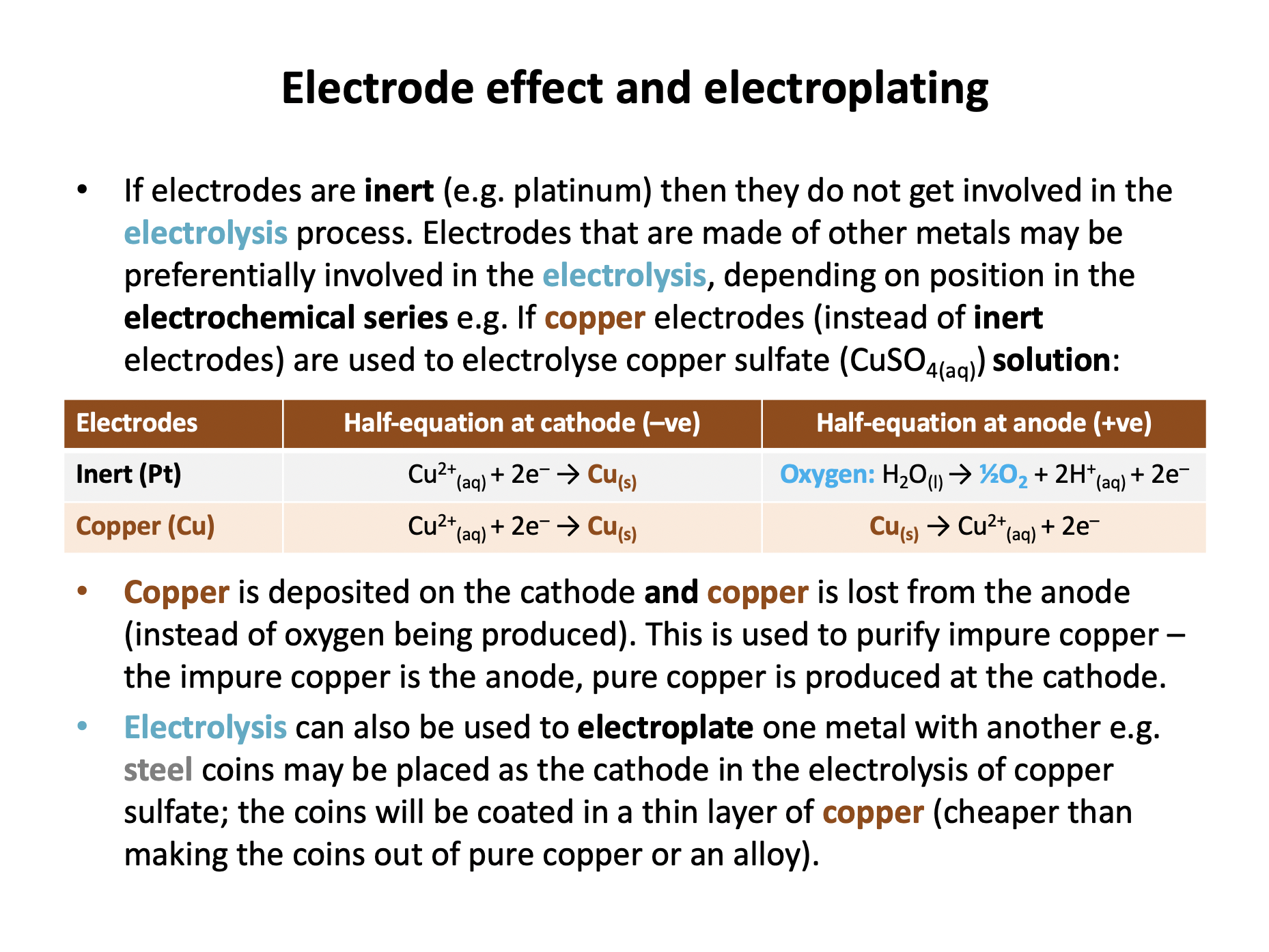
Which half-equations occur at the negative and positive electrodes respectively when aqueous copper chloride solution is electrolysed using copper electrodes?
The species discharged at the negative electrode, gaining e– will be lower (less –ve) in the electrochemical series; copper is lower than water so copper ions are discharged.
Normally the species discharged at the positive electrode, losing e–, will be higher (less +ve) in the electrochemical series. Note! Exception: Chloride ions (½Cl2(g) + e– ⇌ Cl–(aq) Eo = +1.36 V) are preferentially discharged over water (½O2(g) + 2H+(aq) + 2e– ⇌ H2O(l) Eo = +1.23 V) at the positive electrode despite their Eo values (this is a kinetic effect that needs remembering).
However, if a copper electrode is used instead of an inert electrode, copper from the electrode will lose electrons preferentially over chloride ions or water (Cu2+(aq) + 2e– ⇌ Cu(s) Eo = +0.34 V) because its Eo is higher in the electrochemical series.
Thus negative: Cu2+(aq) + 2e– → Cu(s) positive: Cu(s) → Cu2+(aq) + 2e– is the correct answer.
What is produced at the negative and positive electrodes respectively when aqueous copper(II) chloride solution is electrolysed using inert electrodes?
The species discharged at the negative electrode, gaining e– will be lower (less –ve) in the electrochemical series; copper is lower than water so copper ions are discharged.
The species discharged at the positive electrode, losing e–, will be higher (less +ve) in the electrochemical series. Note! Exception: Chloride ions (½Cl2(g) + e– ⇌ Cl–(aq) Eo = +1.36 V) are preferentially discharged over water (½O2(g) + 2H+(aq) + 2e– ⇌ H2O(l) Eo = +1.23 V) at the positive electrode despite their Eo values (this is a kinetic effect that needs remembering).
Thus copper and chlorine is the correct answer.
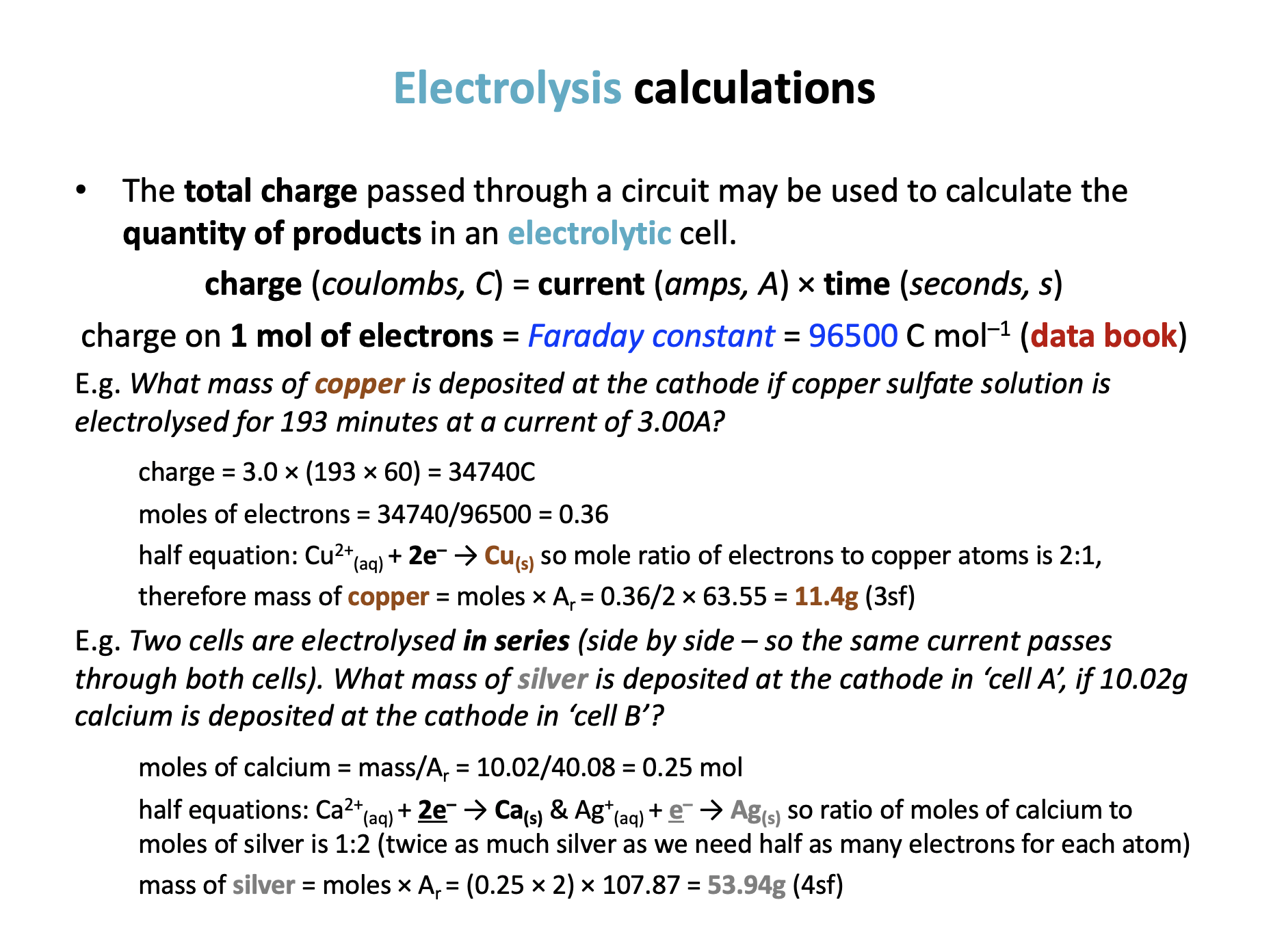
Calculator question: What mass of zinc (in g to 3 sf) is deposited at the cathode if zinc sulfate solution is electrolysed for 30.0 minutes at a current of 5.00A?
Charge = current × time = 5.0 × (30.0 × 60) = 9000C
Moles of electrons passed through circuit = charge/Faraday = 9000/96500 = 0.0932642
Half equation: Zn2+(aq) + 2e– → Zn(s) so mole ratio of electrons to zinc atoms is 2:1,
Therefore mass of zinc = moles × Ar = 0.0932642/2 × 65.38 = 3.0488083 = 3.05g (3sf)
Therefore 3.05 is the correct answer.
Incorrect answers
6.10 uses a 1:1 mole ratio for electrons to atoms instead of 2:1
0.0508 uses 150C of charge (forgetting to multiply minutes by 60 to get seconds)
0.102 uses 150C of charge (forgetting to multiply minutes by 60 to get seconds) and uses a 1:1 mole ratio for electrons to atoms instead of 2:1
Calculator question: Two cells are electrolysed in series. What mass of calcium (in g to 3 sf) is deposited at the cathode in ‘cell A’, if 1.62g aluminium is deposited at the cathode in ‘cell B’?
Moles of aluminium = mass/Ar = 1.62/26.98 = 0.0600444 mol
Half equations: Al3+(aq) + 3e– → Al(s) and Ca2+(aq) + 2e– → Ca(s) so ratio of moles of electrons for aluminium to moles of electrons for calcium is 3:2.
This means that the ratio of moles of aluminium:calcium will be 2:3 (since the same number of electrons will pass through the circuit, but that will give more calcium atoms as only two electrons are needed for each atom, rather than 3 for aluminium).
Moles of calcium = 0.0600444 × 3/2 = 0.0900666
Mass of calcium = moles × Ar = 0.0900666 × 40.08 = 3.6098693 = 3.61g (3sf)
Therefore 3.61 is the correct answer.
Incorrect answers
1.60 uses a 3:2 mole ratio for moles of aluminium:calcium instead of 2:3
2.41 uses a 1:1 mole ratio for moles of aluminium:calcium instead of 2:3
4.81 uses a 1:2 mole ratio for moles of aluminium:calcium instead of 2:3
Paper 1
Core (SL&HL): Redox core (SL and HL) paper 1 questions
AHL (HL only): Redox AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Redox core (SL & HL) paper 2 questions
AHL (HL only): Redox AHL (HL only) paper 2 questions
How much of Electrochemical cells II have you understood?












 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn