 This section of the course, including mass spectrometry, infra-red spectroscopy and nuclear magnetic resonance spectrometry is easier to cope with if we have a really strong grasp of organic chemistry. In particular, a thorough knowledge of functional groups and nomenclature. It is important to learn what to expect from the spectra, and the best way to do this is through practice; the Q bank has lost of spectra in it!
This section of the course, including mass spectrometry, infra-red spectroscopy and nuclear magnetic resonance spectrometry is easier to cope with if we have a really strong grasp of organic chemistry. In particular, a thorough knowledge of functional groups and nomenclature. It is important to learn what to expect from the spectra, and the best way to do this is through practice; the Q bank has lost of spectra in it!
Ensure you are confident using the terms below and learn the asterisked* definitions
Index of hydrogen deficiency, Infrared spectrum, Fingerprint region, Mass spectrum, Molecular ion (M+), Fragment ions, Hydrogen environment, TMS, Integration trace, Chemical shift
Flashcards
Topics: 11.3: Spectroscopic identification
Levels: All
Number of questions: 10
Mode: Normal
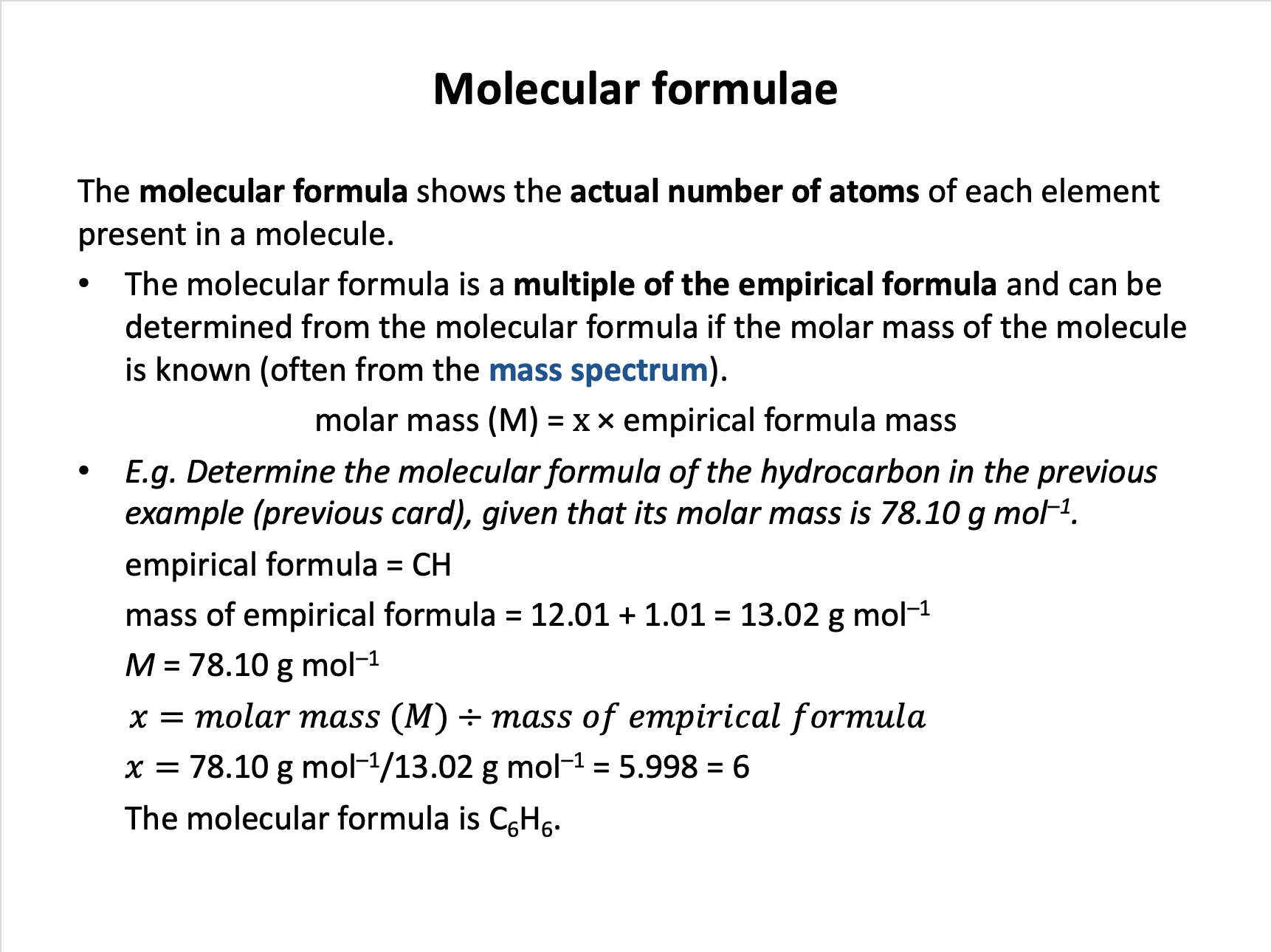
An organic compound consists of 40.0% carbon, 6.7% hydrogen and 53.3% oxygen by mass, and has a molar mass of approximately 60 g mol−1.
What is the molecular formula of the compound?
Find the simplest ratio of the number of (moles of) atoms.
Start by assuming 100g: Thus 40.0g of C atoms, 6.7g of H atoms, 53.3g of O atoms.
| Carbon | Hydrogen | Oxygen | |
moles = mass/molar mass | 40.0/12.01 = 3.330557868 | 6.7/1.01 = 6.633663366 | 53.3/16.00 = 3.33125 |
divide through by smallest | 3.33../3.33.. = 1 | 6.63../3.33.. ≈ 2 | 3.33../3.33.. ≈ 1 |
| whole numbers | 1 | 2 | 1 |
Thus the empirical formula is CH2O.
The empirical 'formula unit' has a mass of approx. 30 (12+2+16), and the molecular mass of the molecule is approx. 60.
60 / 30 = 2
So there are two 'formula units' of the empirical formula in the molecule: The molecular formula is C2H4O2.
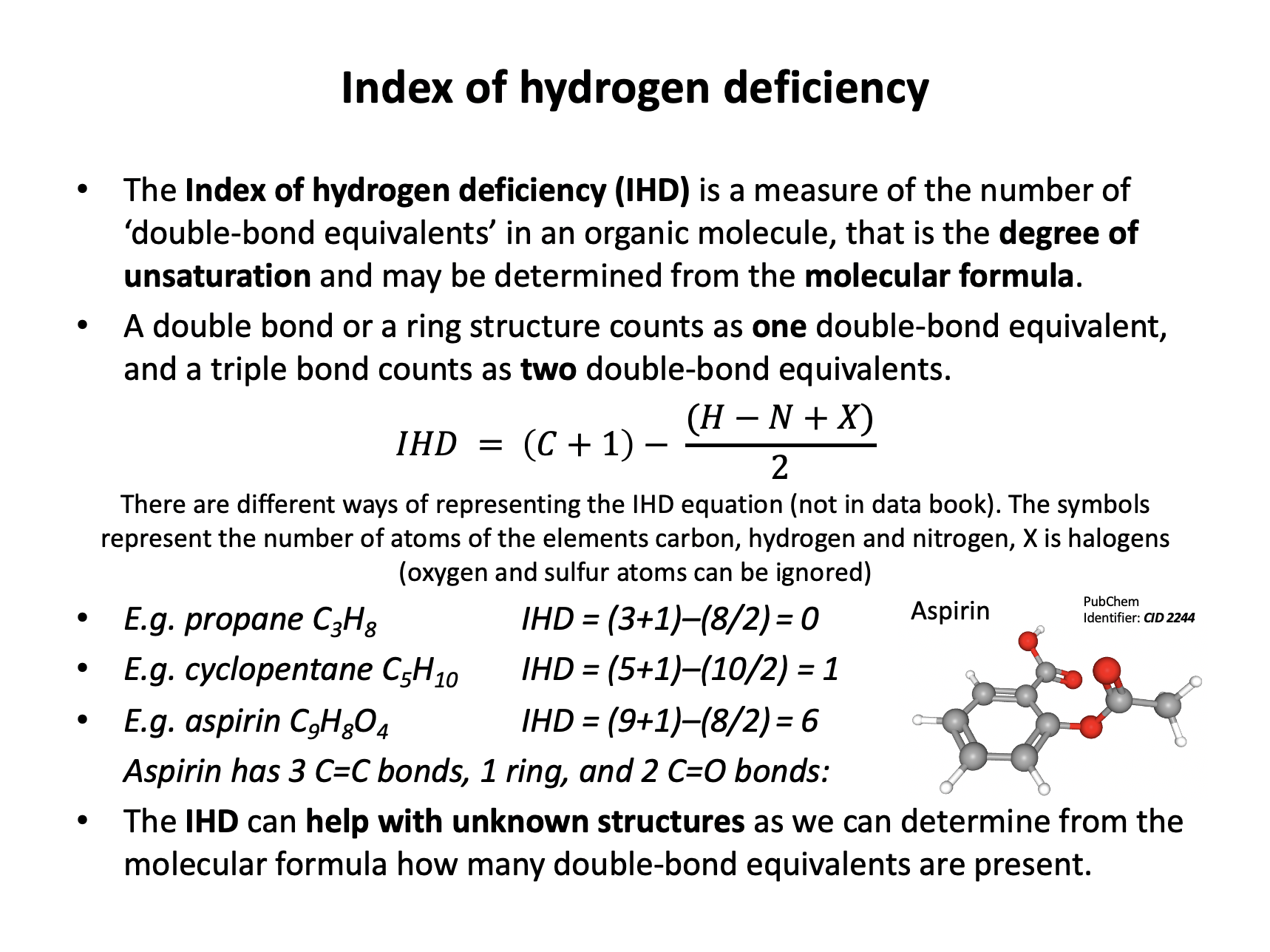
What is the index of hydrogen deficiency for cyclohexene (C6H10)?
The index of hydrogen deficiency is a measure of the 'double bond equivalents' in a molecule. When given a simple molecule like cyclohexene, it is possible to work out the IHD without the equation; the double bond counts as one and the ring counts as another one, so the correct answer is 2.
The equation can be used:
\(IHD = (C+1)-( {H-N+X \over 2})\)
IHD = (6+1) – (10/2) = 7 – 5 = 2
And also gives an answer of 2.
What is the index of hydrogen deficiency for paracetamol (acetaminophen) (C8H9NO2)?
The index of hydrogen deficiency is a measure of the 'double bond equivalents' in a molecule.
The equation can be used, where the letters represent the appropriate elements and X represents any halogen (oxygen can be ignored):
\(IHD = (C+1)-{(H-N+X) \over 2}\)
IHD = (8+1) – (9–1+0)/2 = 9 – 4 = 5
Therefore the correct answer is 5.
That is four double bonds and one ring:
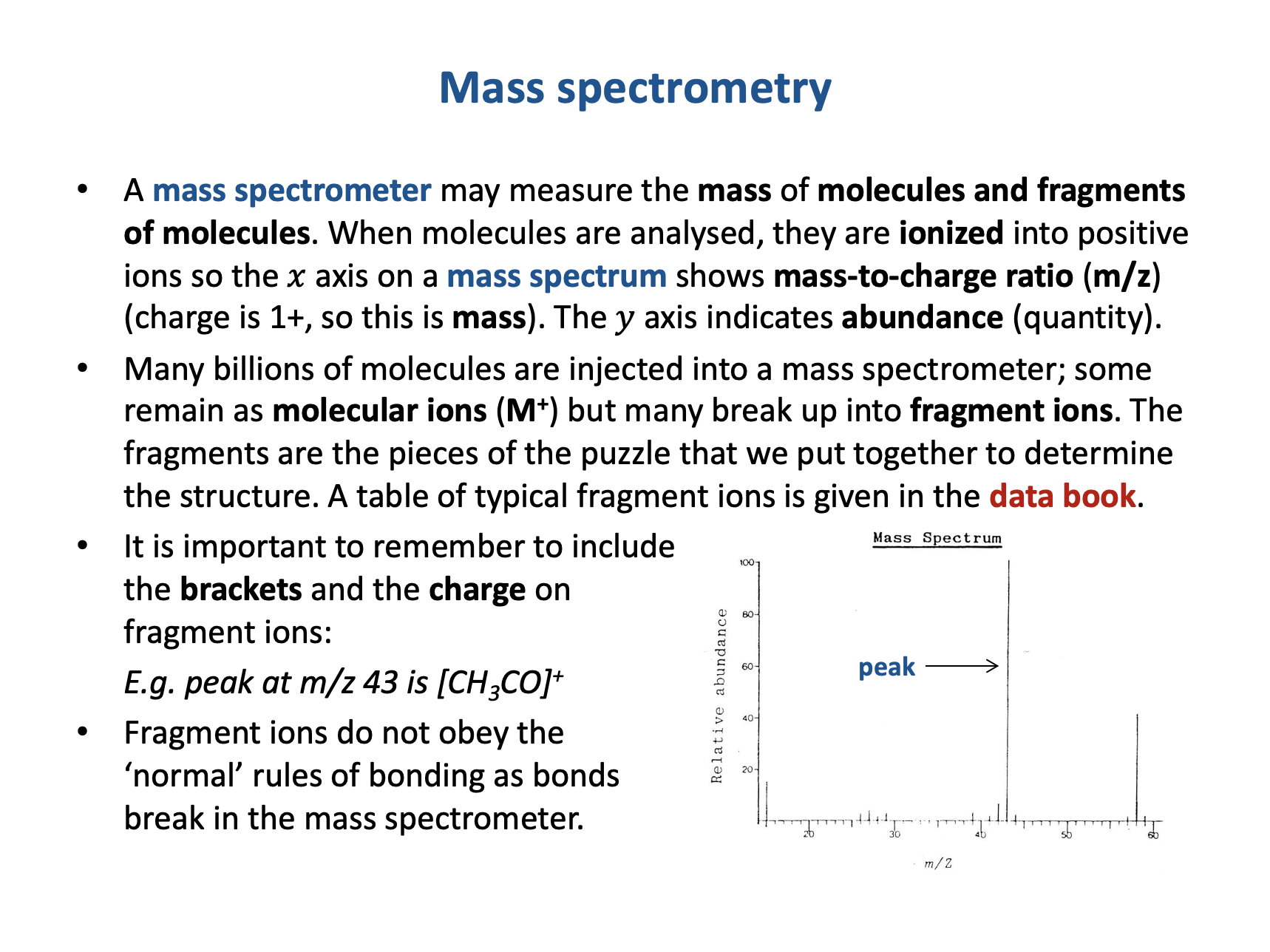
Which of these ions could be responsible for peaks found in the following mass spectrum?
1: [CH3]+
2: [C2H3O]+
3: [C3H6O]+
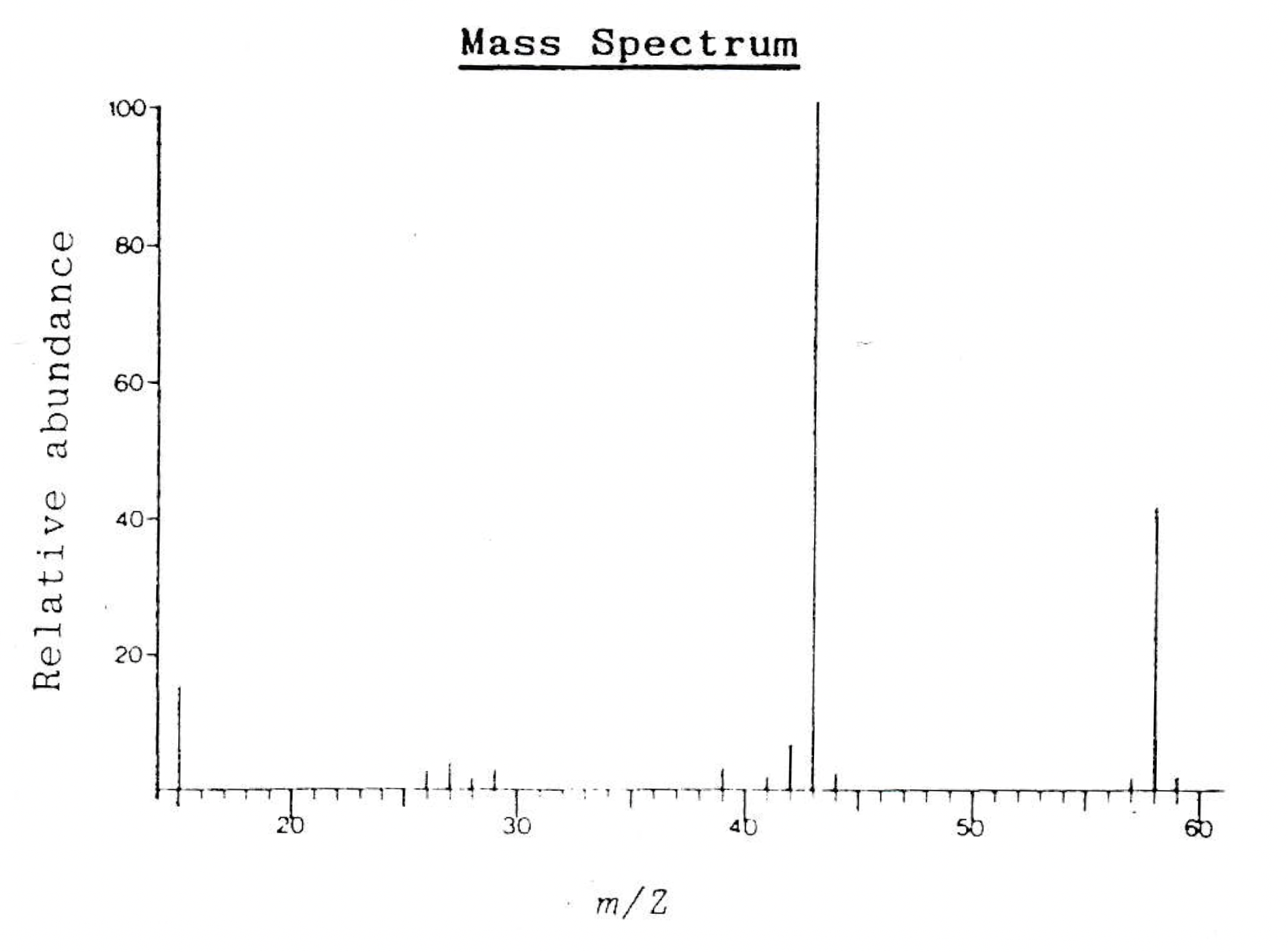
The integer masses of the ions need to be matched to m/z values for peaks in the spectrum:
[CH3]+ (12+3=15) matches the peak at m/z 15.
[C2H3O]+ (24+3+16=43) matches the (large) peak at m/z 43.
[C3H6O]+ (36+6+16=58) matches the molecular ion peak at m/z 58.
Therefore 1, 2 and 3 is the correct answer.
A mass spectrum of an organic compound of molecular formula C5H10O shows a peak at m/z 29.
Which of these ions could be responsible for the peak at m/z 29?
1: [CHO]+
2: [C2H5]+
3: [NCH3]+
All three fragment ions have integer masses of 29 that correspond to a peak at 29 m/z. However [NCH3]+ contains nitrogen which is not given in the molecular formula, so [NCH3]+ cannot be resonsible for the peak.
The compound C5H10O (we are not given any information on structure) could however fragment to give both [CHO]+ and [C2H5]+.
Therefore 1 and 2 only is the correct answer.
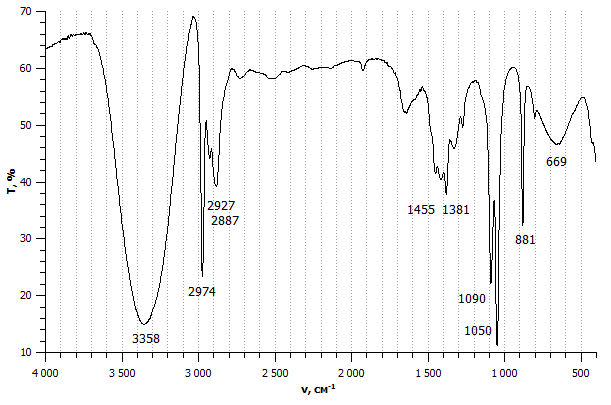
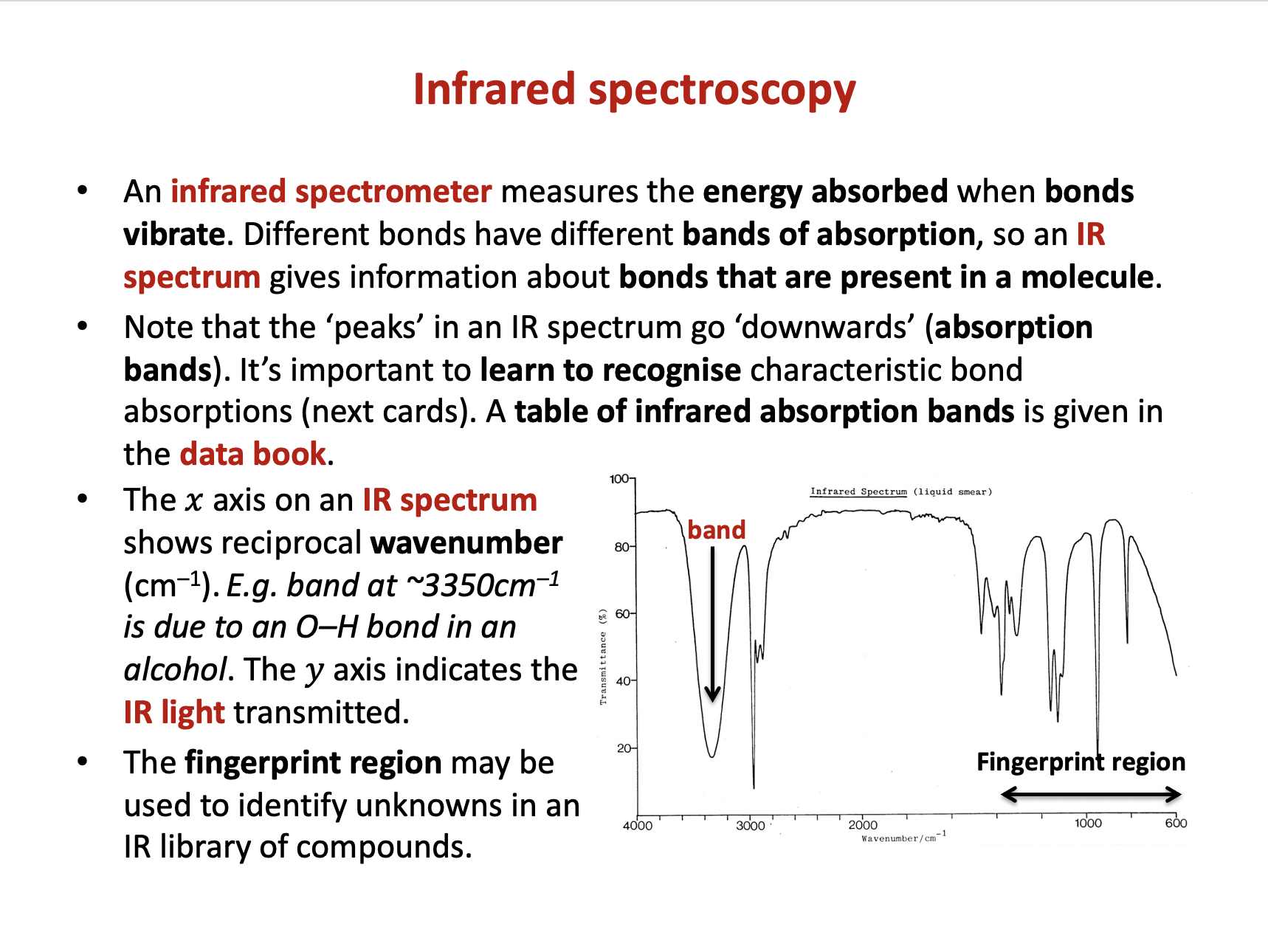
An infrared spectrum of compound X is shown below.
Which of these bond/s is/are likely to be present in compound X?
1: O−H
2: C=C
3: C−H
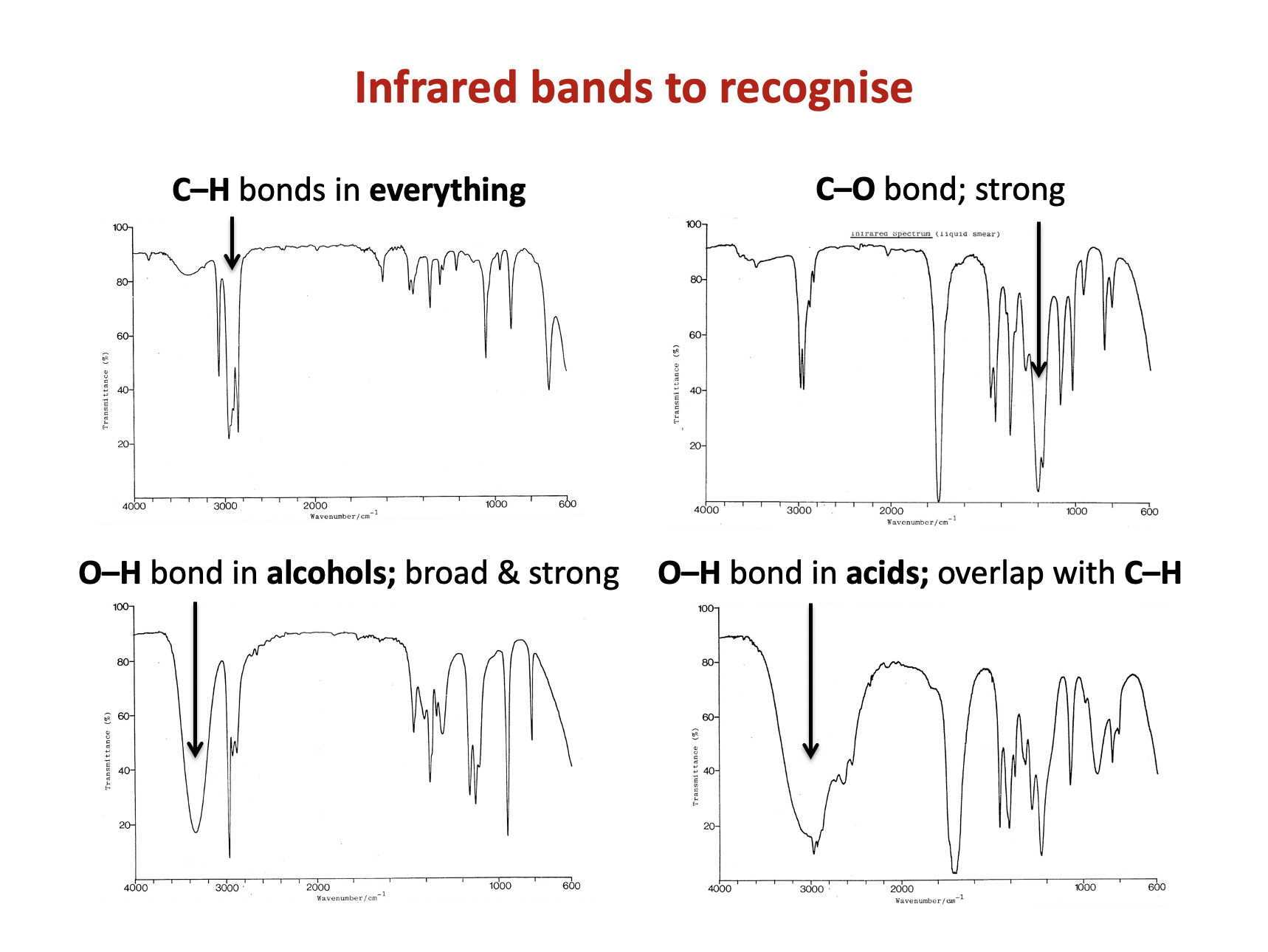
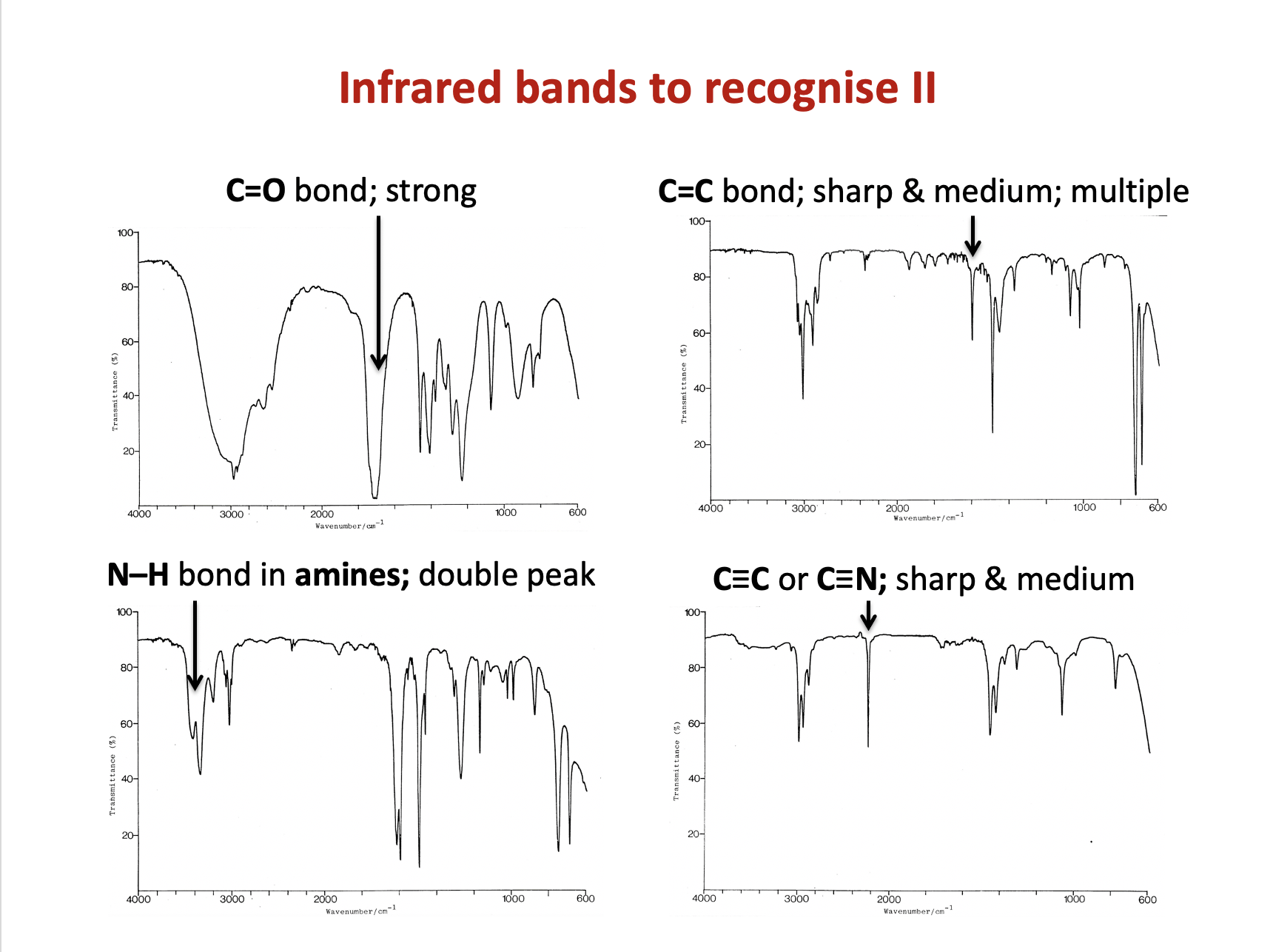
The data book contains a table of IR data, but it's important to learn to recognise characteristic bond absorption bands (see revision cards).
The broad and strong band at 3350cm−1 indicates an O−H bond.
The (always present) collection of bands at approx. 3000 cm−1 indicate C−H bonds.
However, there is not a sharp, medium band that one might expect to see approx.1620-1680 cm−1 to indicate a C=C bond.
Therefore 1 and 3 only is the correct answer.
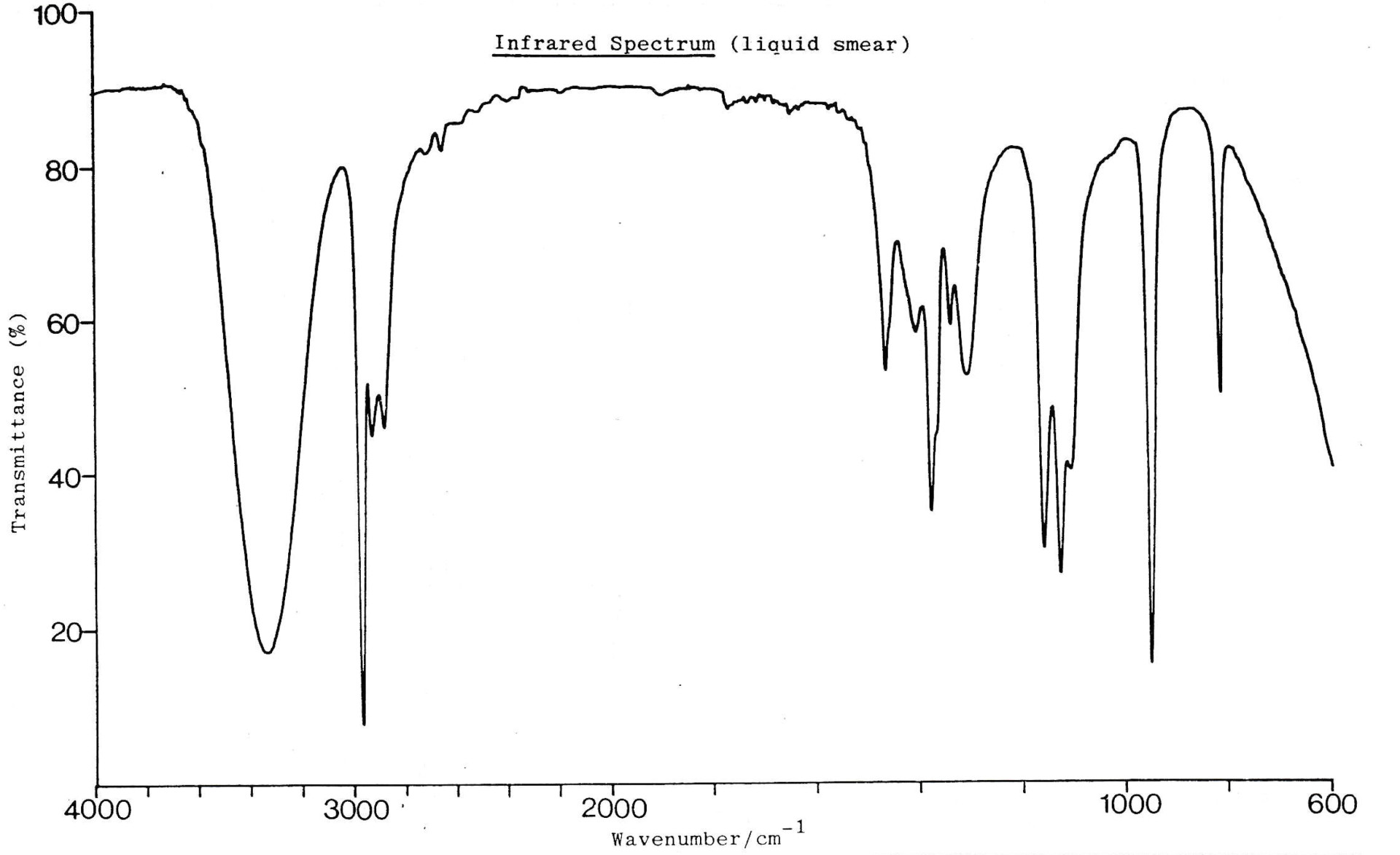
An infrared spectrum of compound Y is shown below.
Which of these bond/s is/are likely to be present in compound Y?
1: O−H
2: C=O
3: C−H
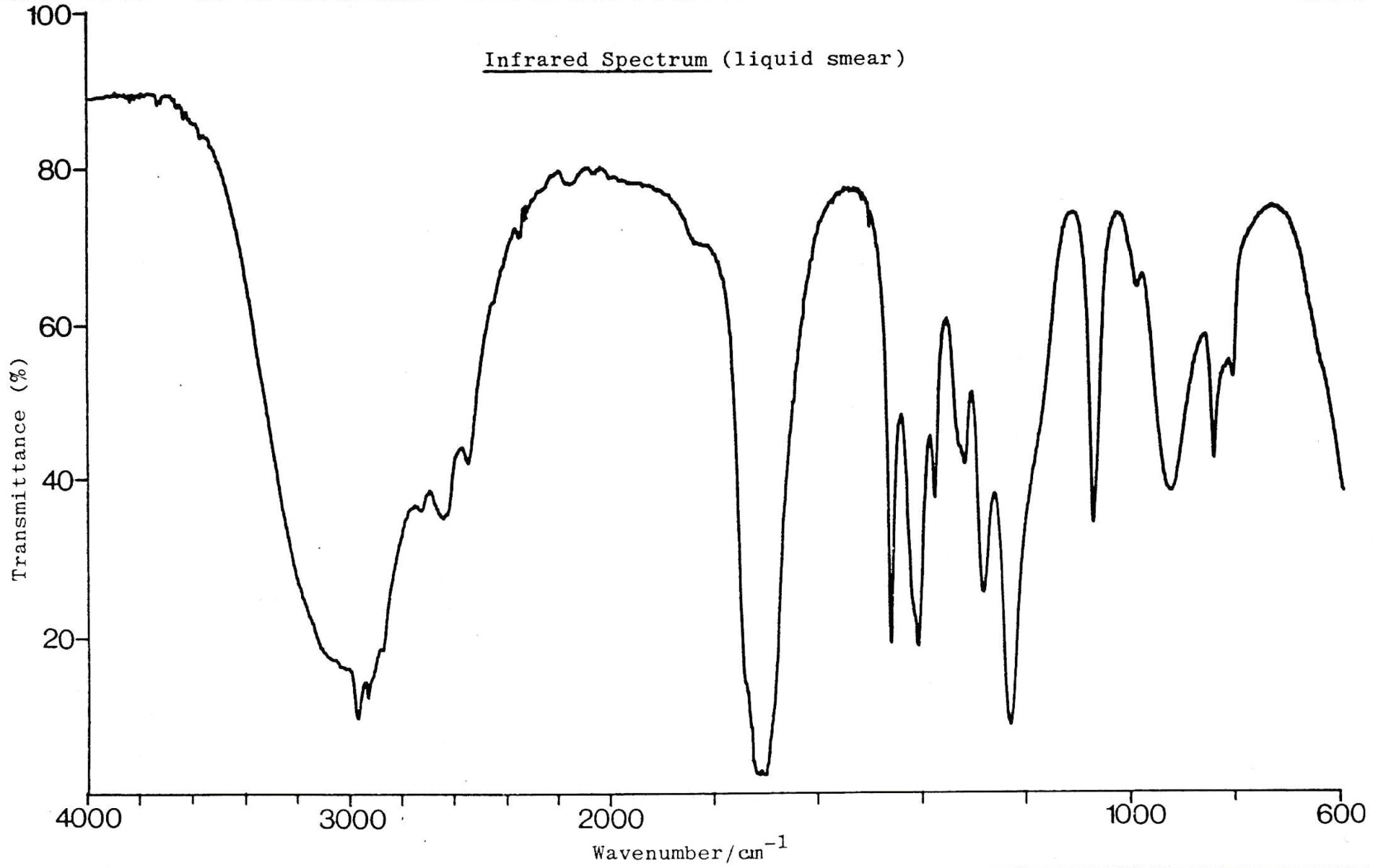
The data book contains a table of IR data, but it's important to learn to recognise characteristic bond absorption bands (see revision cards).
There are two bands overlapping here at 3000 cm−1.
The broad and strong band at approx. 3000cm−1 indicates an O−H bond in a carboxylic acid, and this overlaps with the (always present) collection of bands at approx. 3000 cm−1 that indicate C−H bonds.
There is also a strong band at approx.1750 cm−1 to indicate a C=O bond (as you might expect in a carboxylic acid).
Therefore 1, 2 and 3 is the correct answer.
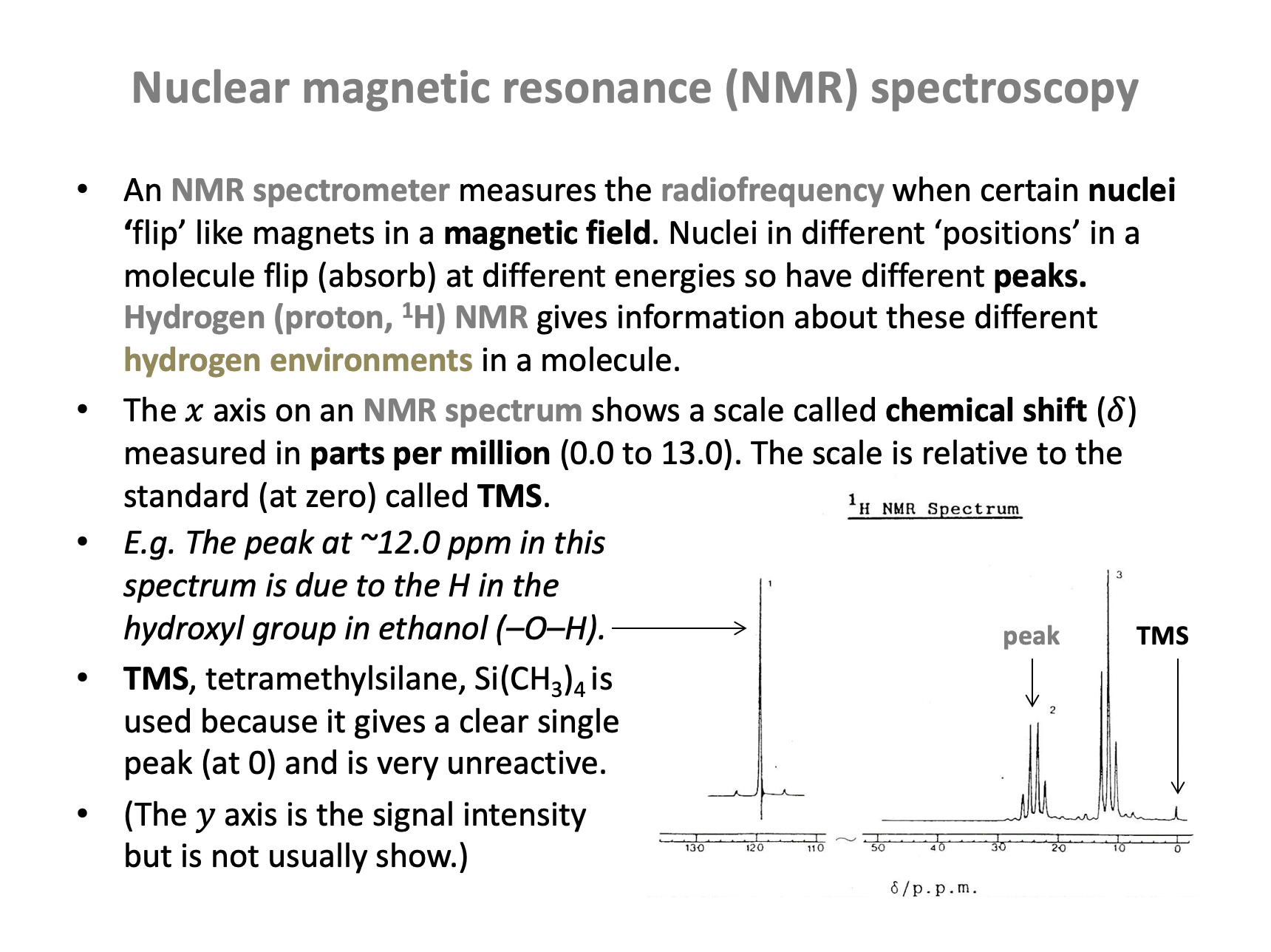
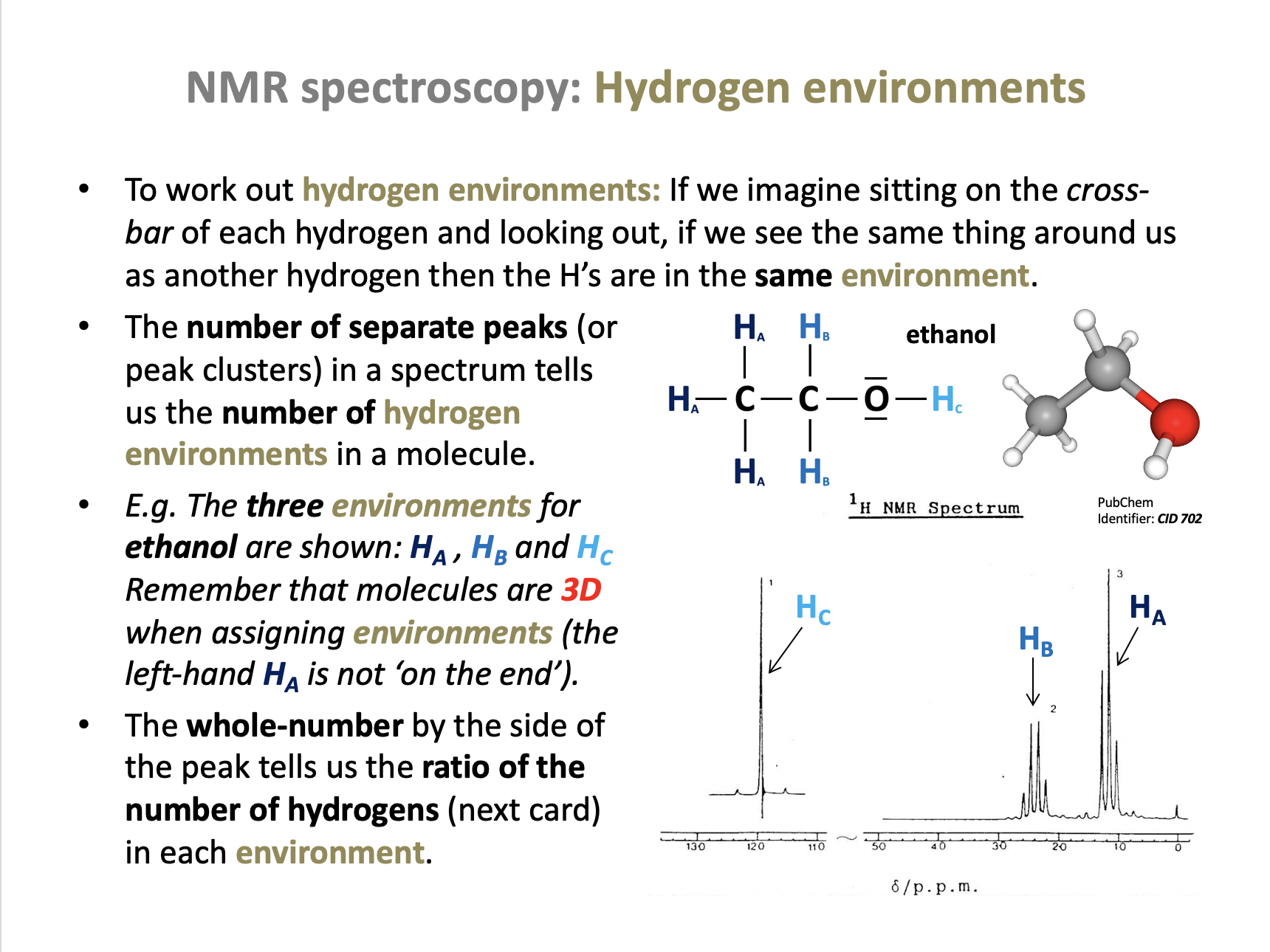
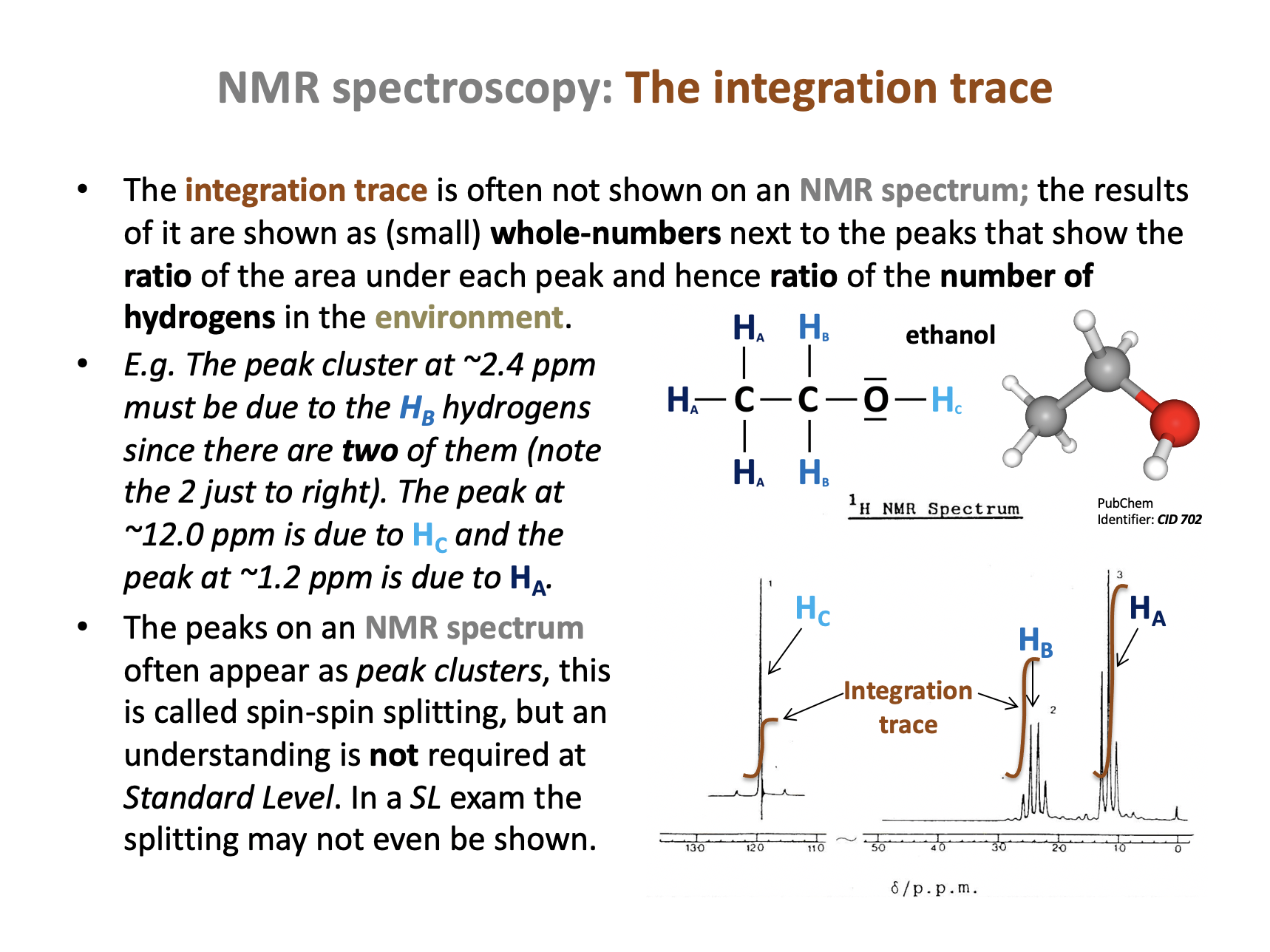
What do the integration trace values of a set of H-NMR peaks represent?
The integration trace value, given as a number by each peak in an NMR spectrum tells us the ratio of the area under the peak, which is the ratio of the number of hydrogens in the environment. This will often be the same as the number of hydrogens, but it is given in the simplest ratio (which can be misleading).
Therefore The ratio of the number of hydrogen atoms in each environment is the correct answer.
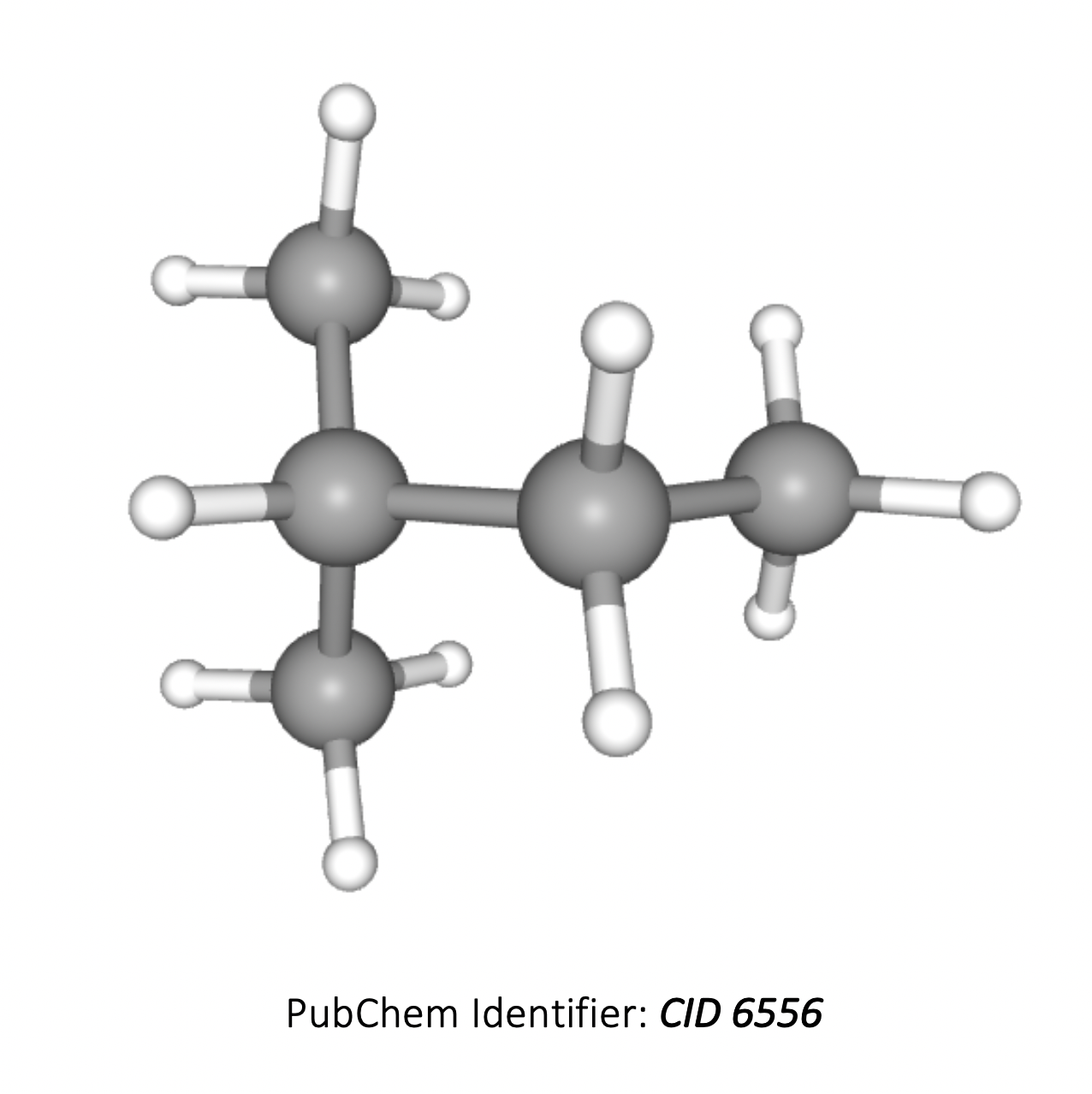
In an H-NMR spectrum of the compound below, how many different hydrogen environments and therefore peaks will be present?
(CH3)2CHCH2CH3
The compound is 2-methylbutane (3D image below). The hydrogens in the two CH3 groups to the left are equivalent, so form one environment. The hydrogens in the CH, CH2 and the CH3 group on the right all contain hydrogens which are in different environments, that is another three environments.
Therefore there are 4 different hydrogen environments in total.
An H-NMR spectrum of compound Z is shown below.
Which of the following organic compounds could be compound Z?
1: Propanone, CH3COCH3
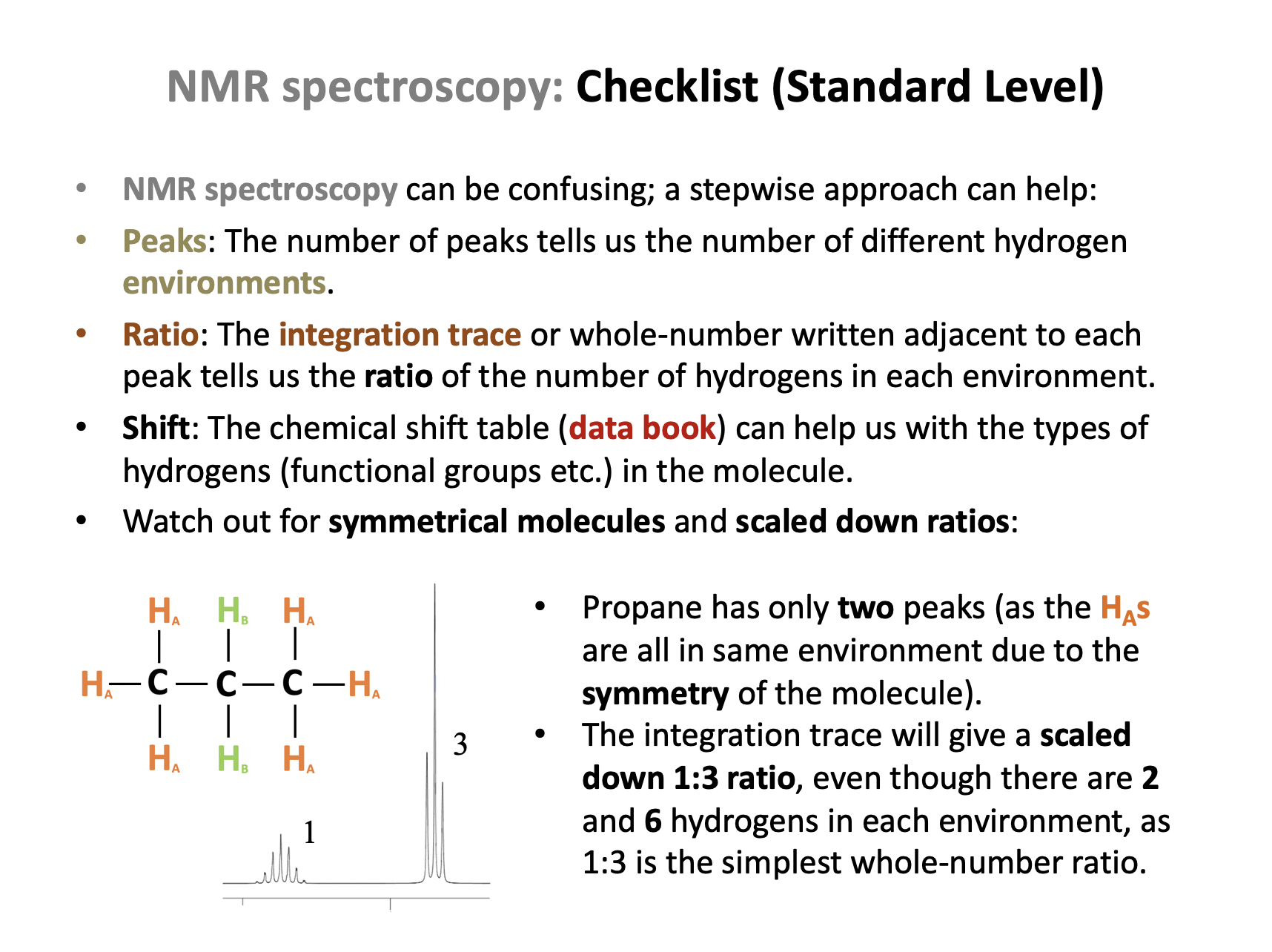
2: Propane, CH3CH2CH3
3: Propanol, CH3CH2OH
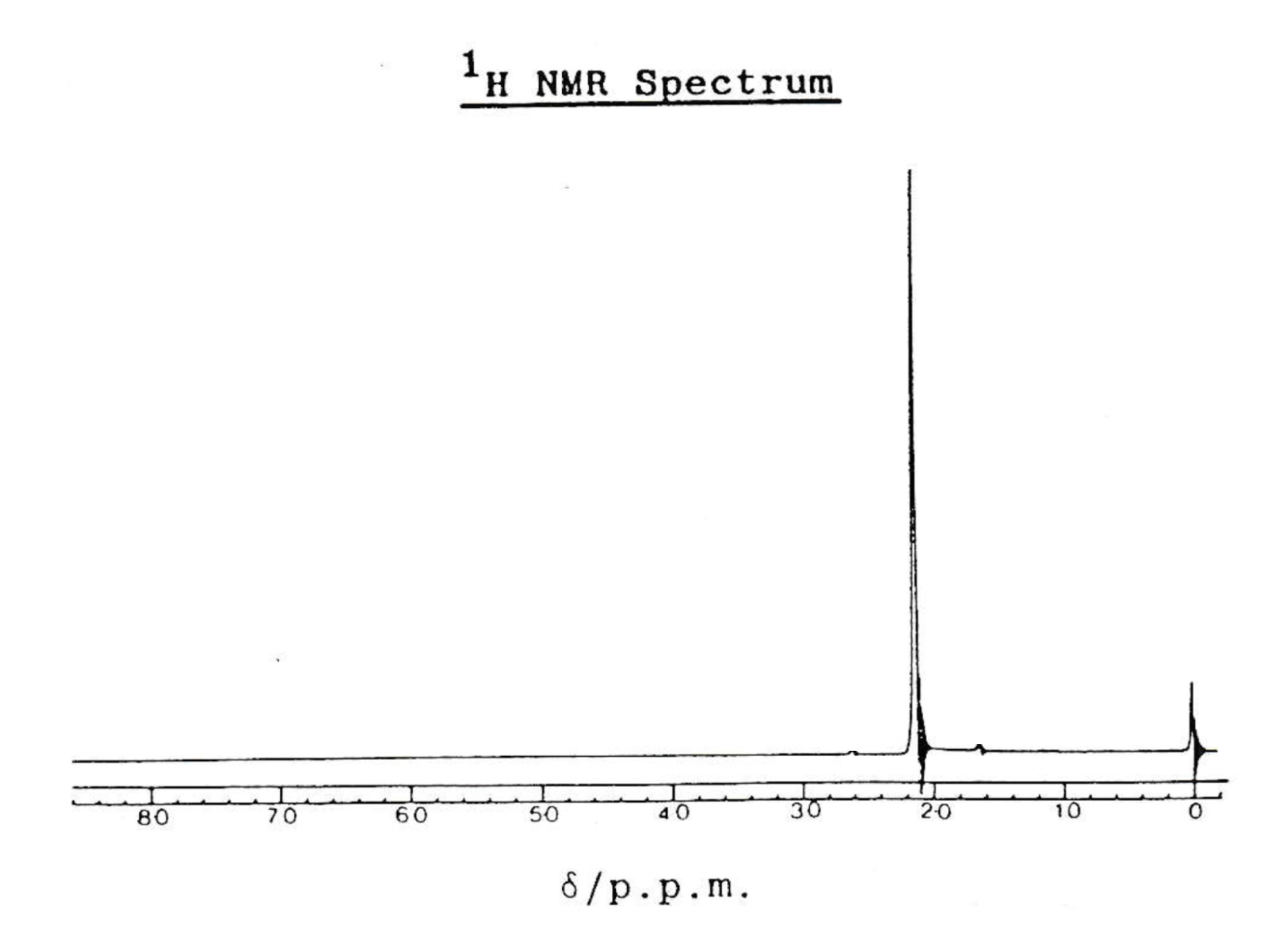
The H-NMR spectrum shows only one peak and therefore compound Z must have only one hydrogen environment.
Propanone is symmetrical; the hydrogens in the two CH3 groups are identical. Therefore, propanone has only one hydrogen environment and will produce one peak in its H-NMR spectrum.
Propane is symmetrical too, but although the hydrogens in the two CH3 groups are identical, the CH2 group in the middle contains hydrogens in a second environment. Propane will produce two peaks in its H-NMR spectrum.
Propanol is not symmetrical. The hydrogens in the CH3, CH2 and OH groups are all in different environments, so propanol will produce three peaks in its H-NMR spectrum.
Therefore 1 only is the correct answer.
Paper 1
Core (SL&HL): Measurement core (SL and HL) paper 1 questions
AHL (HL only): Measurement AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Measurement core (SL & HL) paper 2 questions
AHL (HL only): Measurement AHL (HL only) paper 2 questions
How much of Spectroscopic identification I have you understood?

















 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn