 Don't start revising this section of the course until you are confident with Spectroscopic identification 11.3 first, as this section is mostly an extension of the techniques encountered there. Again, it is easier to cope with analytical chemistry if we have a really strong grasp of organic chemistry. In particular, a thorough knowledge of functional groups and nomenclature.
Don't start revising this section of the course until you are confident with Spectroscopic identification 11.3 first, as this section is mostly an extension of the techniques encountered there. Again, it is easier to cope with analytical chemistry if we have a really strong grasp of organic chemistry. In particular, a thorough knowledge of functional groups and nomenclature.
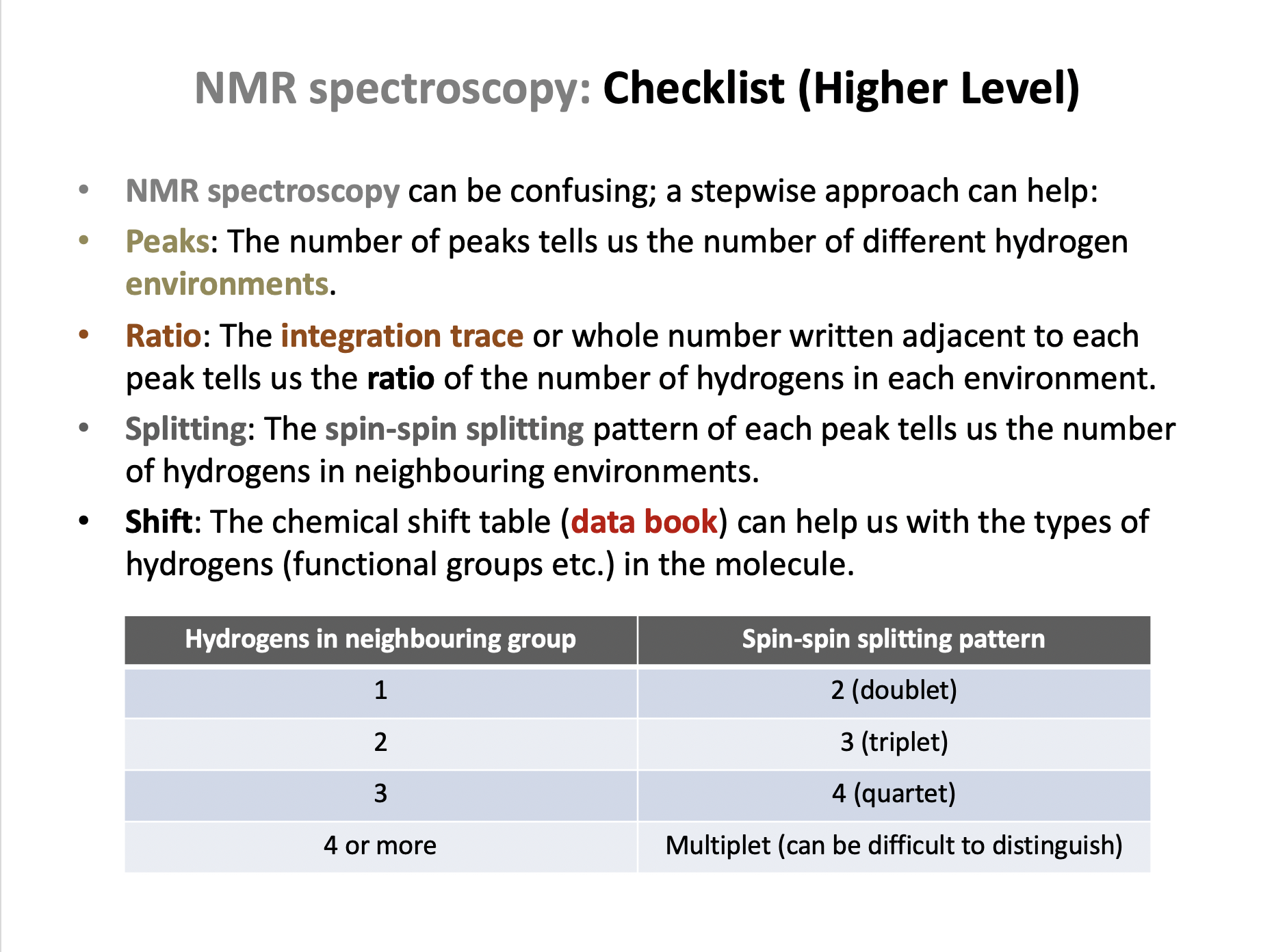
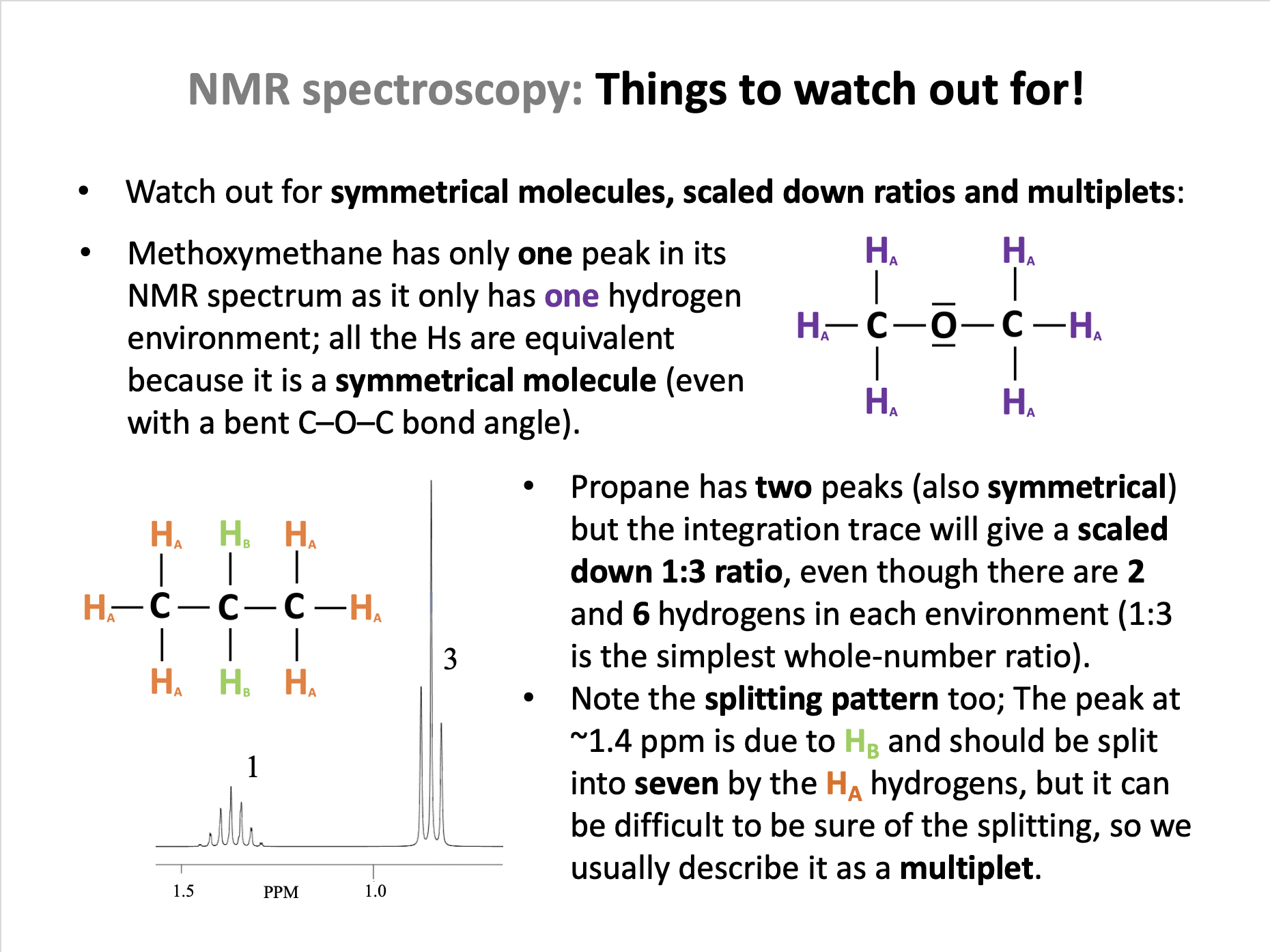
Ensure you are confident using the terms in the section Spectroscopic identification 11.3 and also the terms below
Spin-spin splitting, n+1 rule, X-ray crystallography
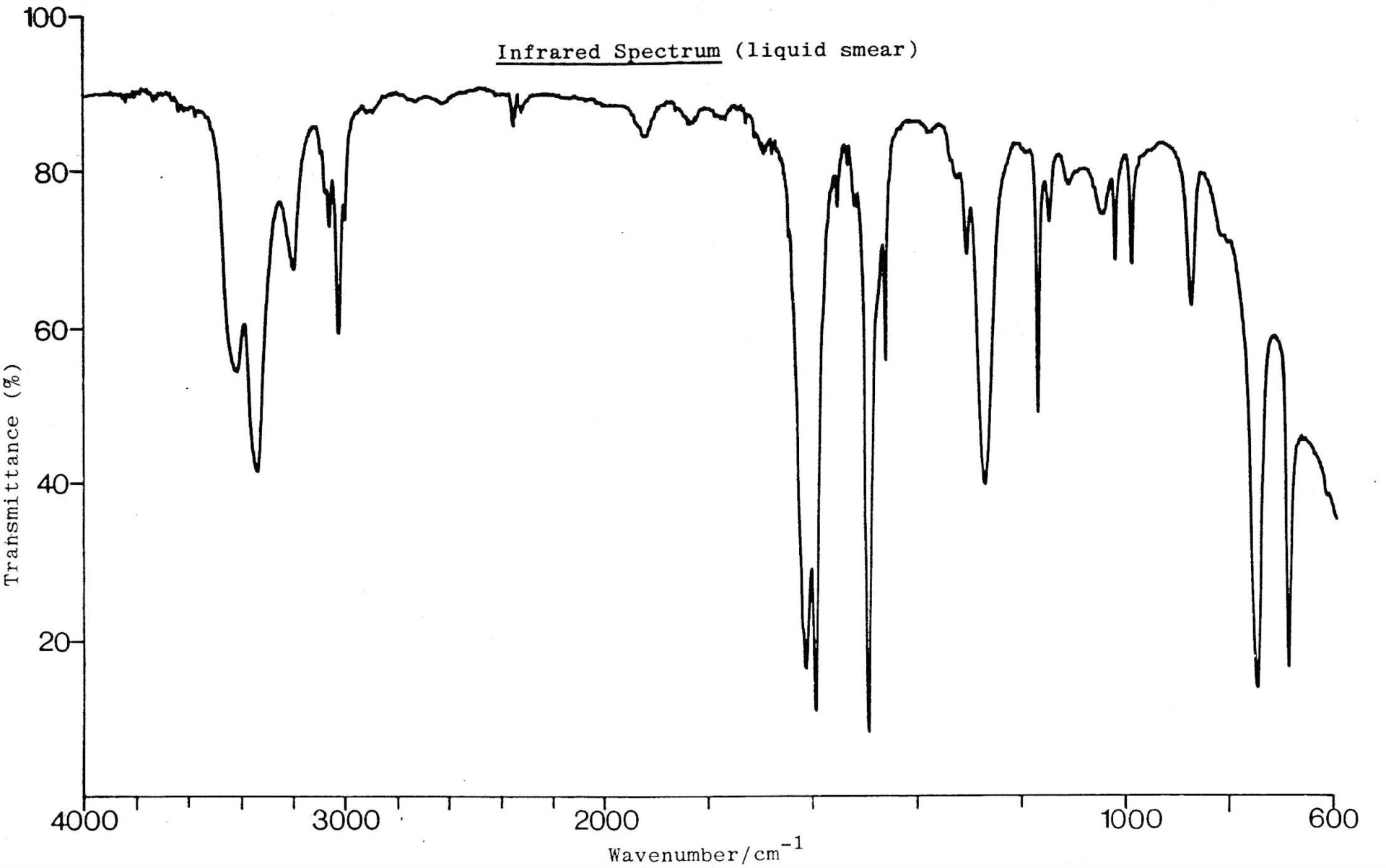
An infrared spectrum of compound H is shown below.
Which of these bond/s is/are likely to be present in compound H?
1: O−H
2: C=C
3: N−H
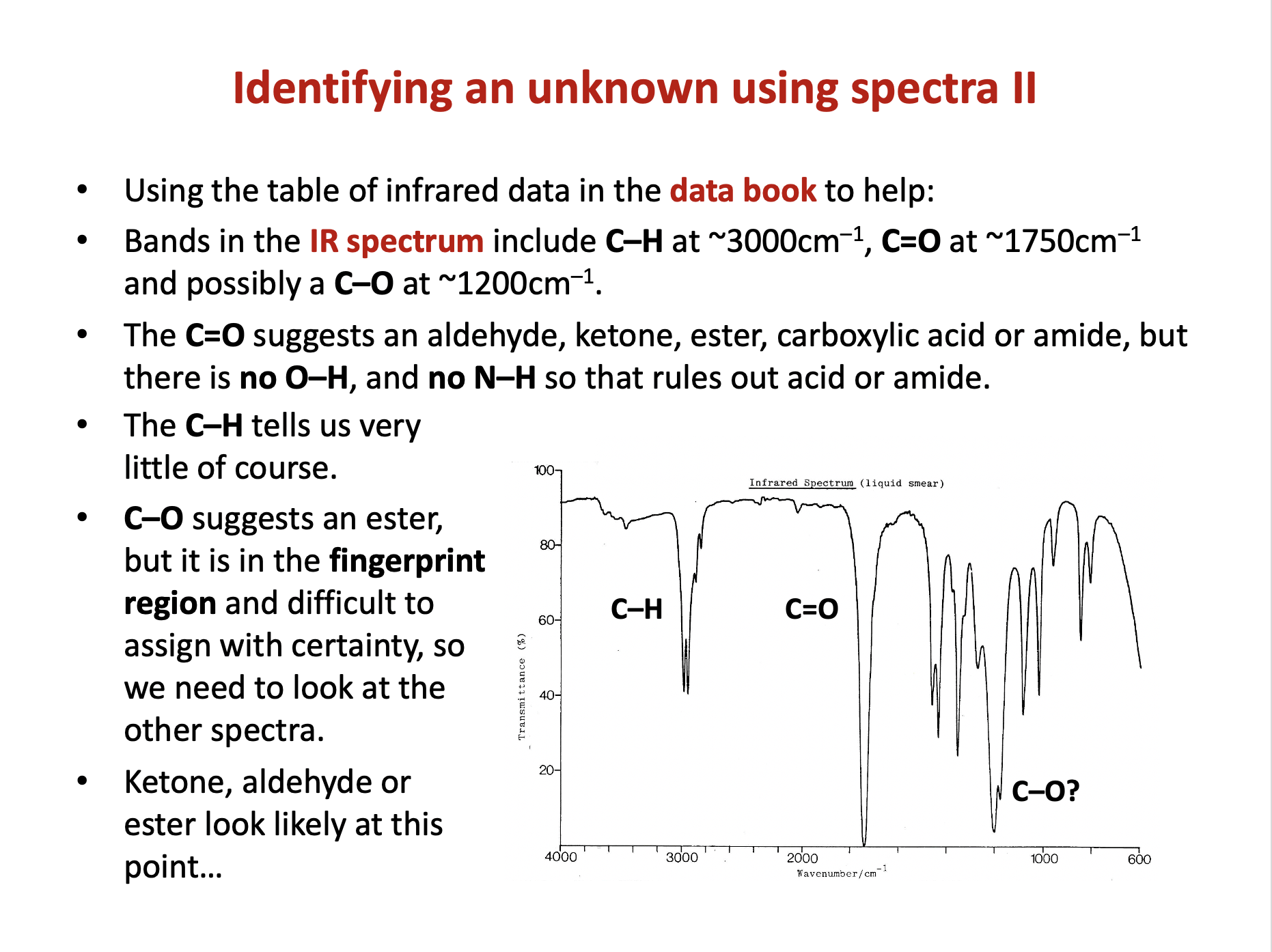
The data book contains a table of IR data, but it's important to learn to recognise characteristic bond absorption bands (see revision cards).
The double 'peak' band at 3300-3400cm−1 indicates an N−H bond. It is characteristic of amines or amides and is not as broad/rounded as an O−H band.
There is no O−H band present.
There is also a double sharp, medium (to strong) band at approx.1600-1650 cm−1 to indicate a C=C bond. C=C bands can often appear as multiple peaks.
Therefore 2 and 3 only is the correct answer.
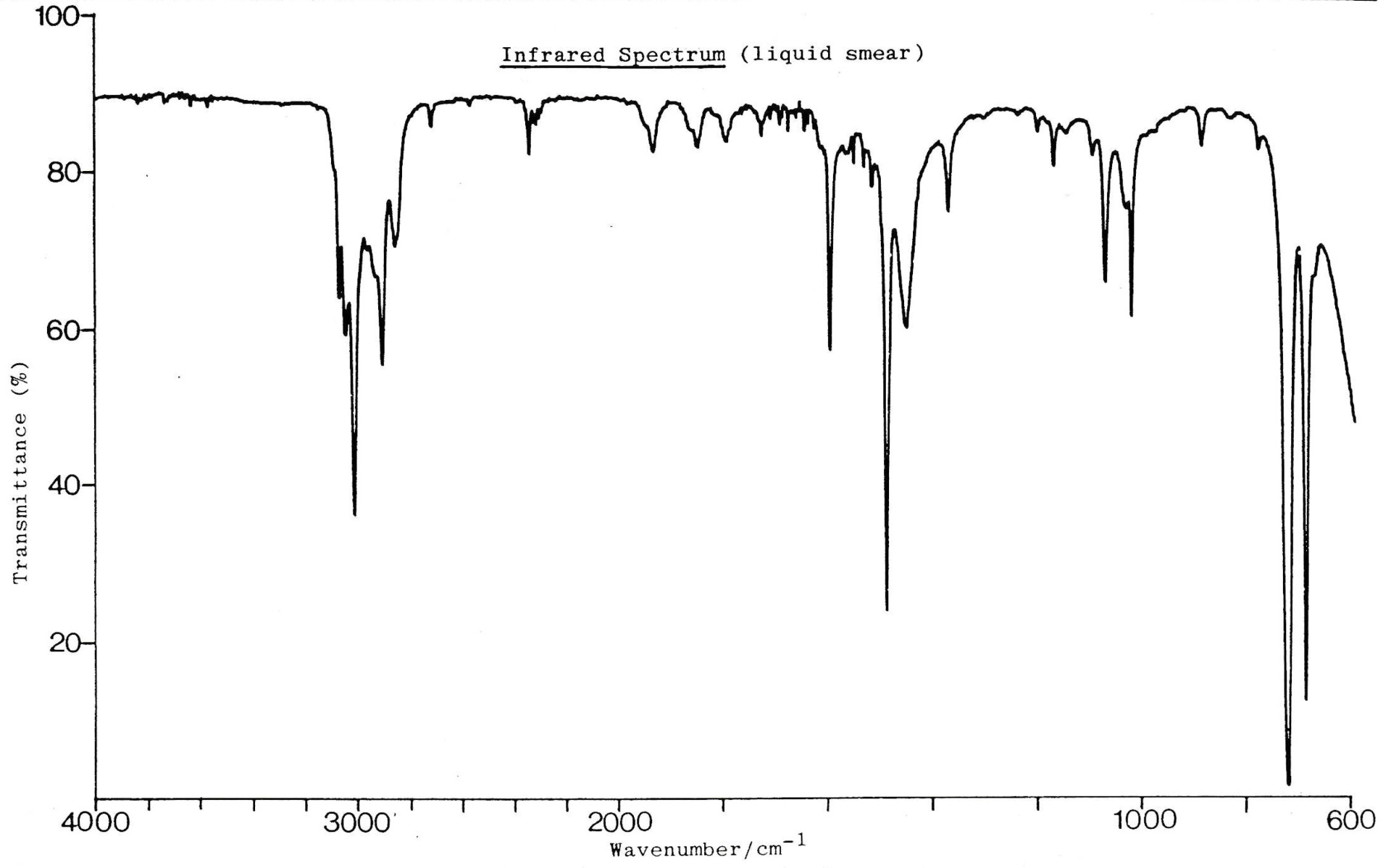
An infrared spectrum of compound Z is shown below.
Which of these could be compound Z?
1: Propene
2: Propane
3: Propanol
The data book contains a table of IR data, but it's important to learn to recognise characteristic bond absorption bands (see revision cards).
There is no O−H band present. If there were, we would see a broad and strong band somewhere between 2500-3500cm−1 . Compound Z could not therefore be propanol.
The (always present) collection of bands at approx. 3000cm−1 indicate C−H bonds. C−H bonds are present in all three compounds given.
There is also a sharp, medium band at approx.1620cm−1 to indicate a C=C bond. C=C bands can often appear as multiple peaks, but this is singular. A C=C bond is present in propene, but not in propane. Compound Z could not therefore be propane.
Compound Z could be propene.
Therefore 1 only is the correct answer.
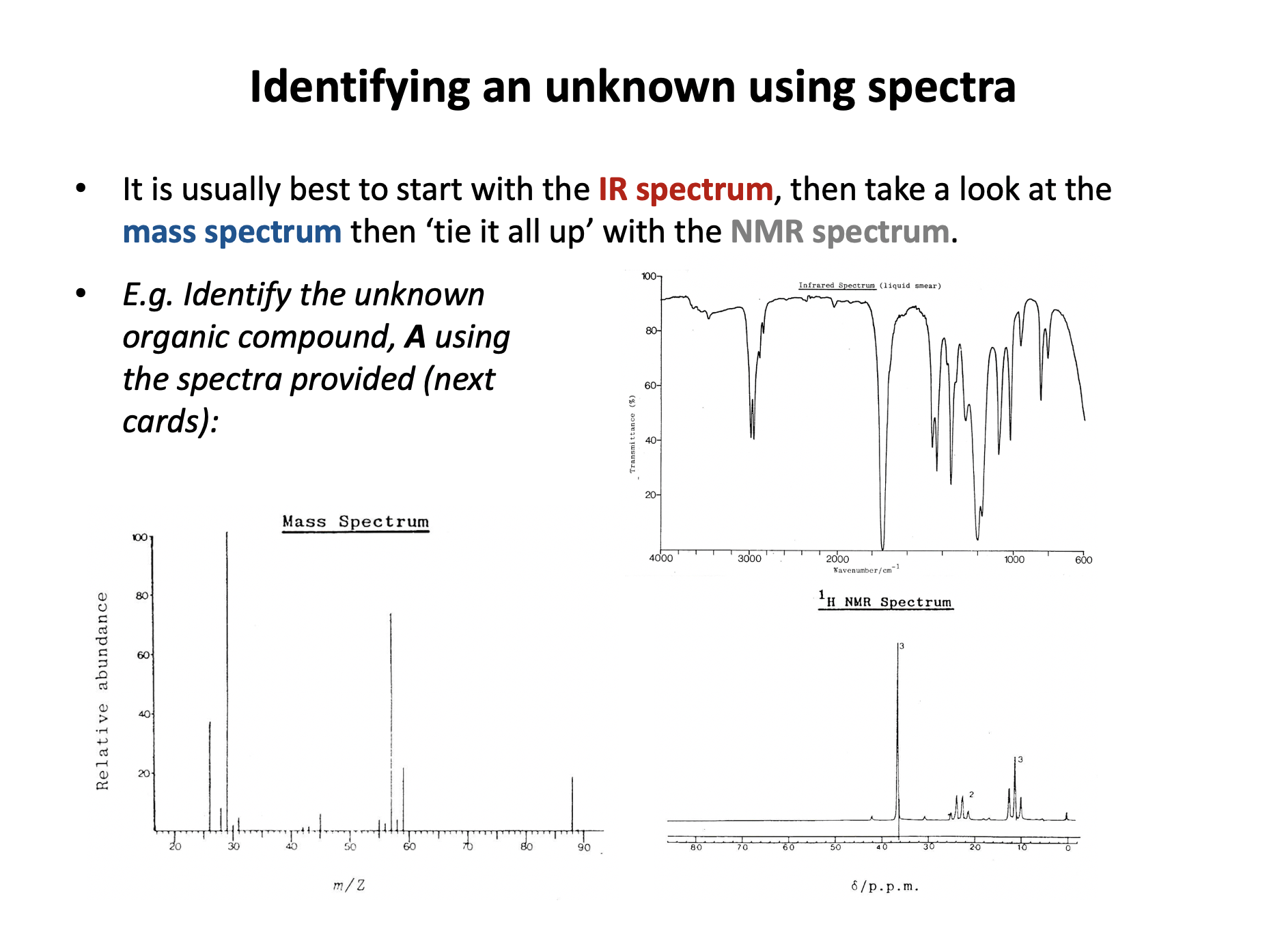
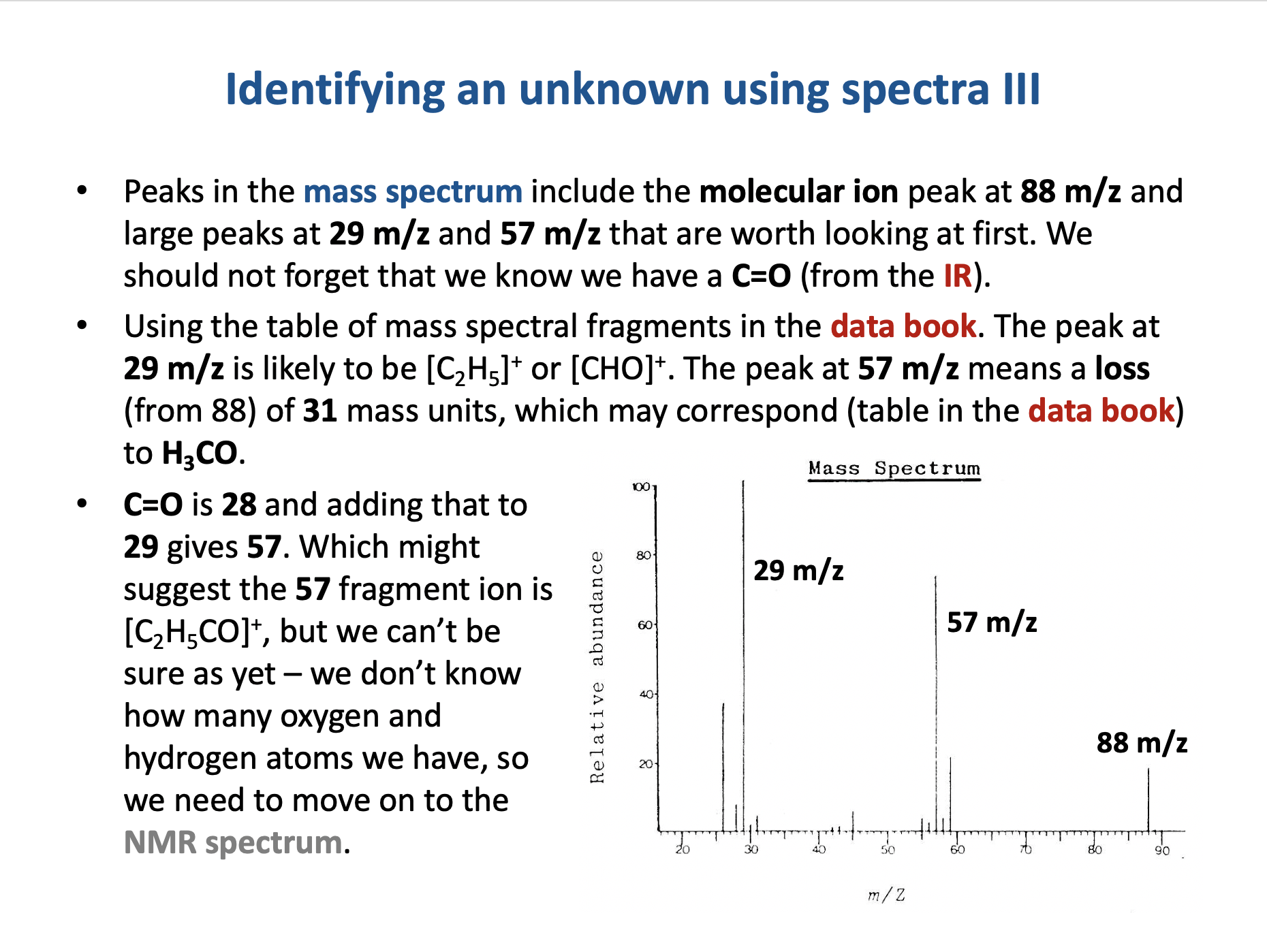
The mass spectrum below is that of compound A.
Compound A also shows a strong band in its IR spectrum at 1750cm−1.
Which of these could be compound A?
1: Butane, C4H10
2: Propanal, CH3CH2CHO
3: Propanone, CH3COCH3
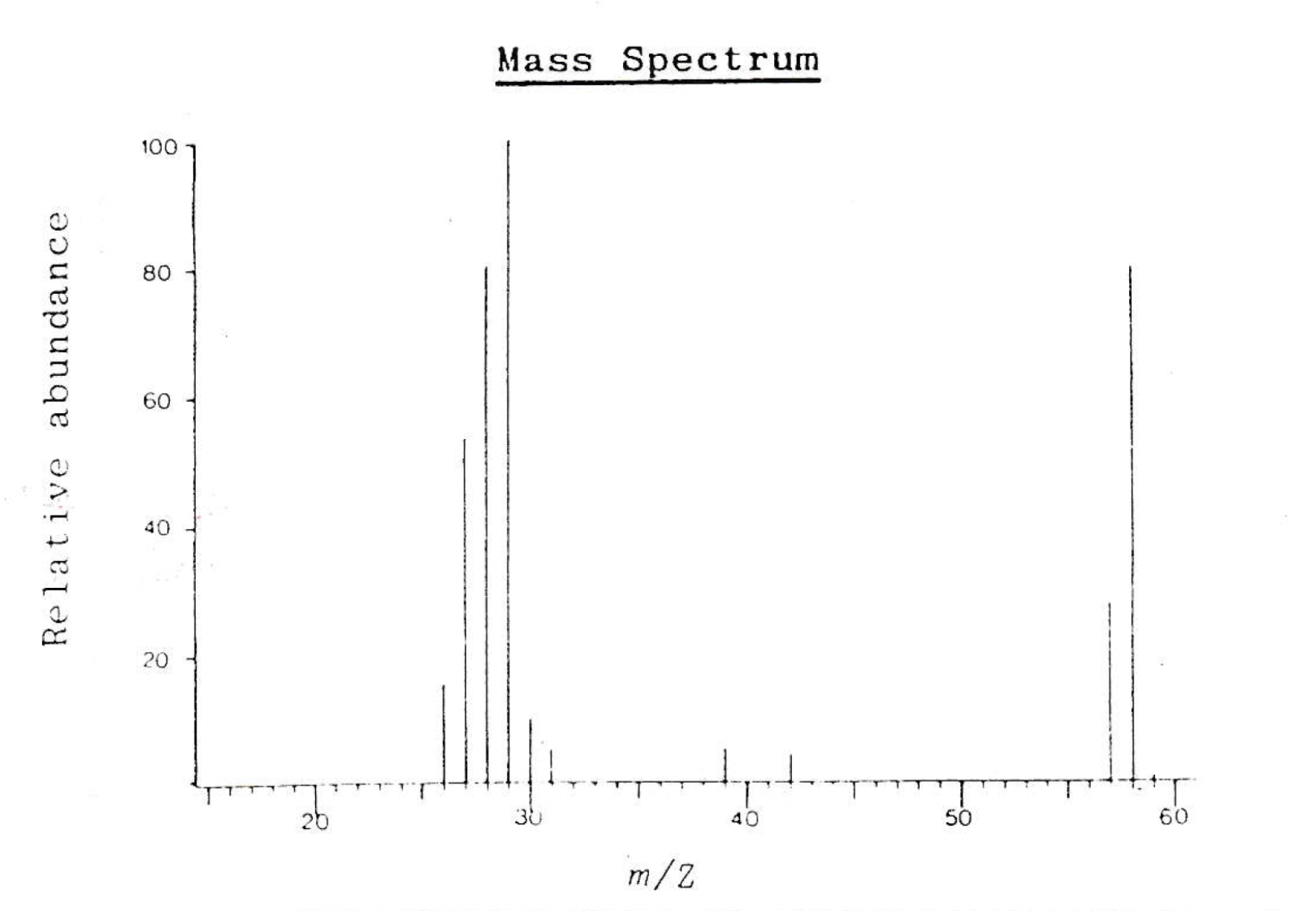
The molecular ion peak in the mass spectrum is at 58 m/z. All three compounds given have an integer mass of 58.
The question states that compound A shows a strong band in its IR spectrum at 1750cm−1. This tells us that it has a C=O bond, so compound A cannot be butane. Both propanal and propanone have a C=O bond.
The largest peak in the mass spectrum is at 29 m/z. This could correspond to fragment ions [C2H5]+ or to [CHO]+, (both noted in the table in the data book) both of which are very likely to be produced by propanal. Propanone would not produce a peak at 29; we would see peaks at 15 and/or 43 m/z.
Compound A must be propanal.
Therefore 2 only is the correct answer.
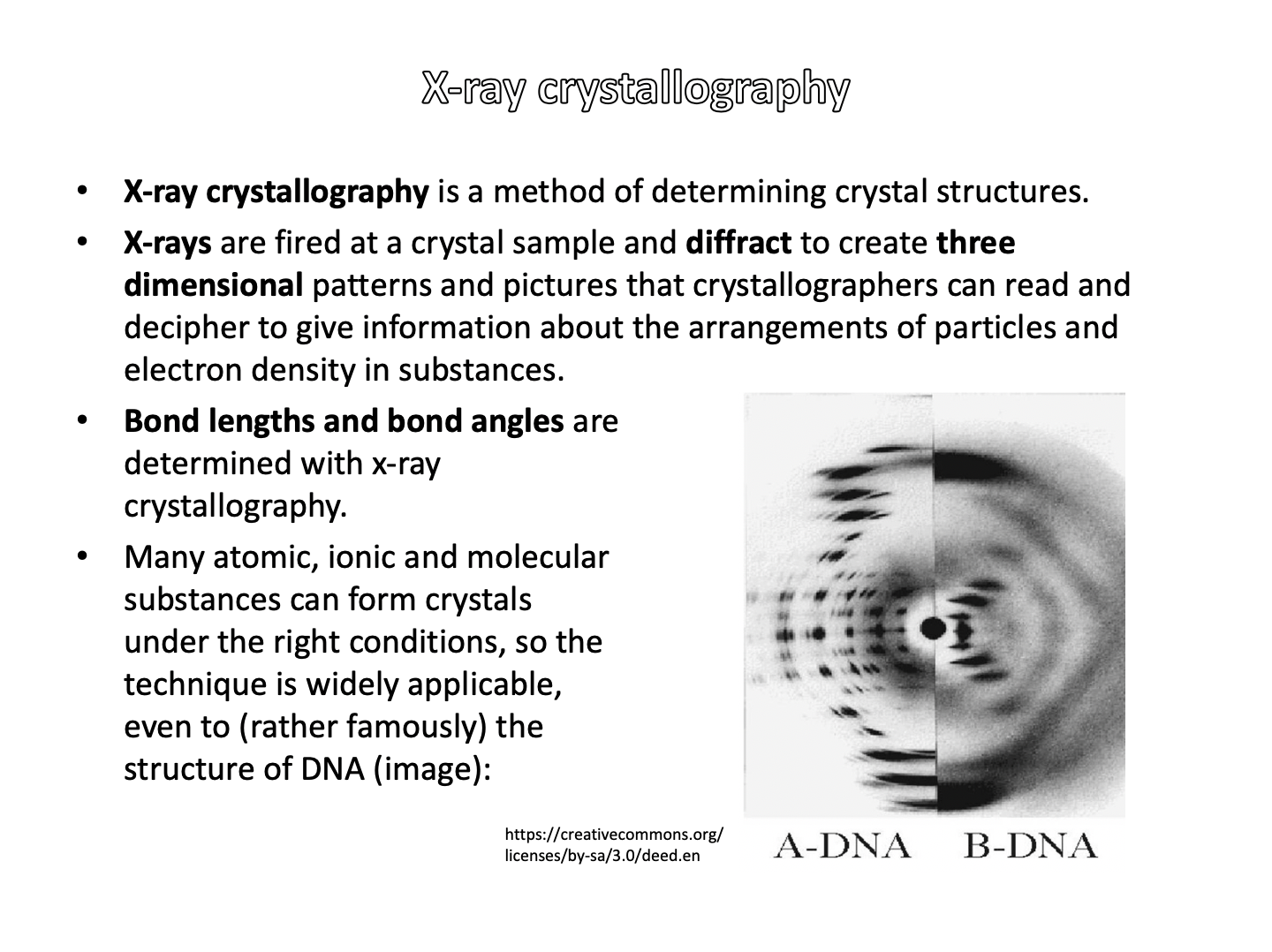
Which of the following techniques might be used to determine bond lengths and bond angles?
X-ray crystallography is a method of determining crystal structures. X-rays are fired at a crystal sample and diffract to create three dimensional patterns and pictures that crystallographers can read and decipher to give information about the arrangements of particles and electron density in substances:
Bond lengths and bond angles are determined with x-ray crystallography.
The other techniques give a lot of information about the structure of organic compounds, but do not tend to give direct information about bond lengths and angles.
X-ray crystallography is the correct answer.
The IR spectrum and NMR spectrum below are both given by compound L.
Which of these could be compound L?
1: Butanenitrile CH3CH2CH2CN
2: Propanoic acid, CH3CH2CO2H
3: Propanamide, CH3CH2CONH2
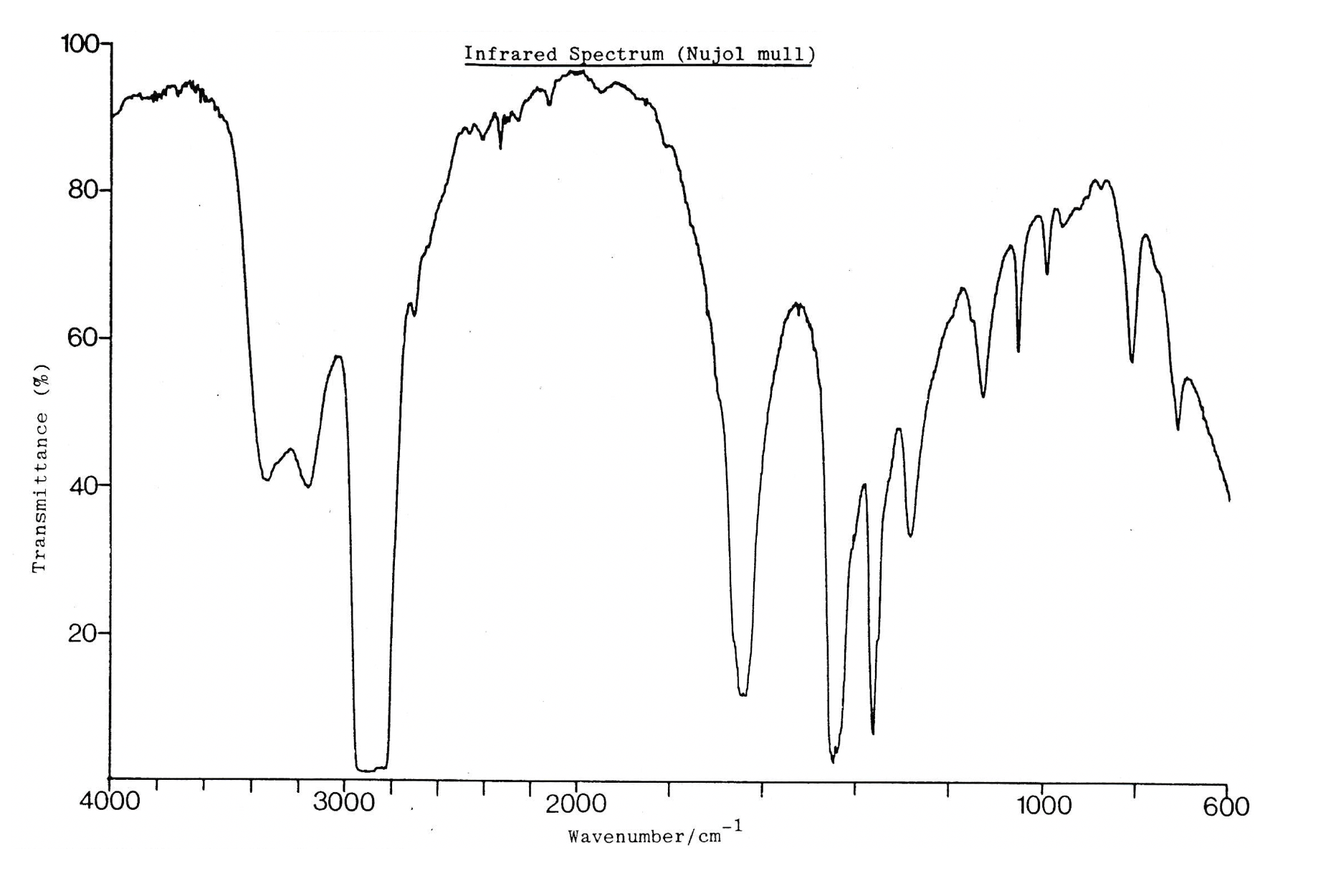
IR spectrum
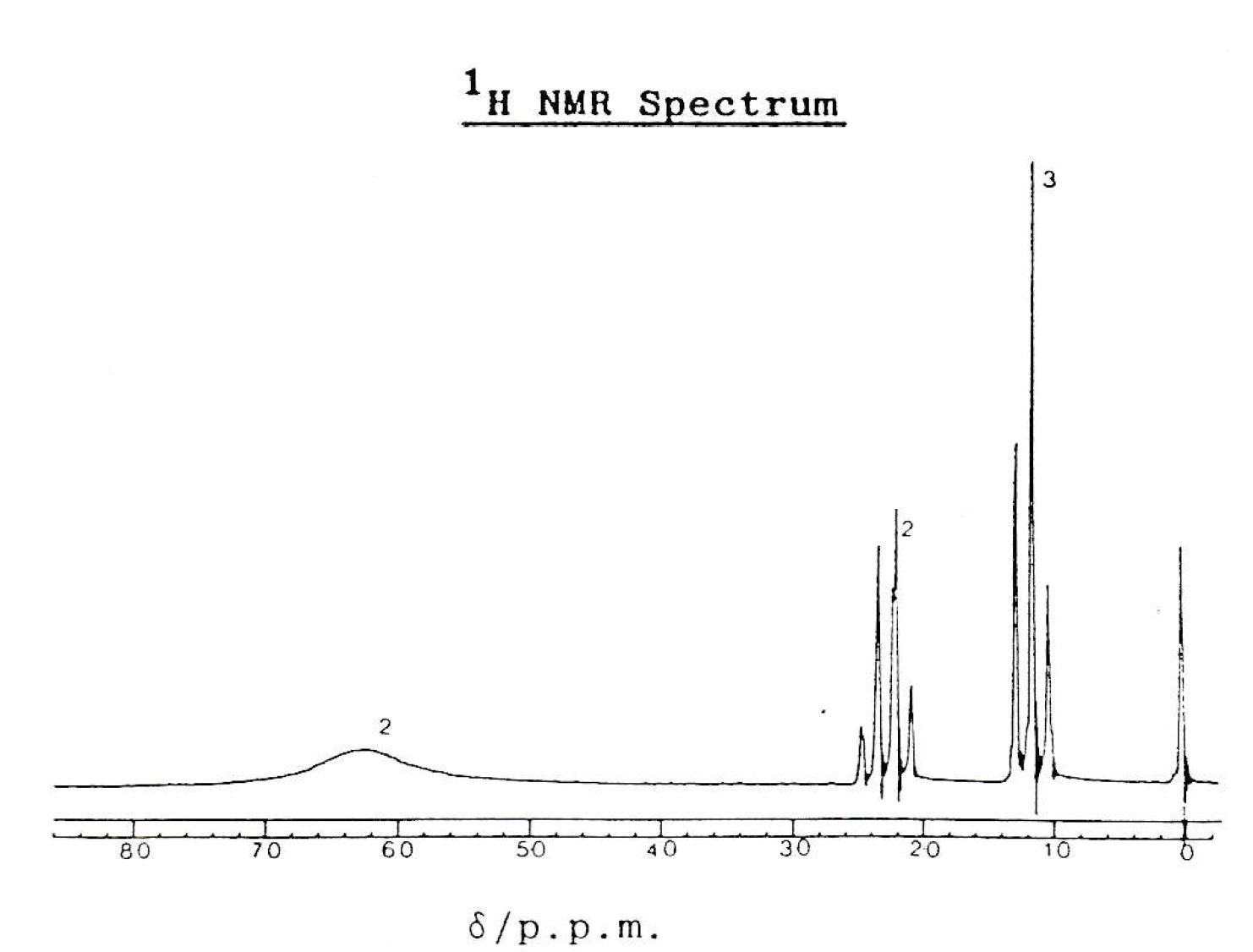
NMR spectrum
The IR spectrum shows a strong band around 1700cm−1. This suggests a C=O bond, so compound L cannot be butanenitrile. Both propanoic acid and propanamide have a C=O bond.
The IR spectrum also shows a characteristic 'double' band at 3300cm−1. This suggests a N−H bond (do not mistake this for an O−H which is broader and more rounded. This would suggest that the compound is an amide (with the C=O).
The NMR spectrum shows three hydrogen environments, with hydrogen ratio of 2:2:3. This fits with propanamide. The splitting pattern also fits propanamide (and does not fit with either of the other two compounds).
Compound L must be propanamide.
Therefore 3 only is the correct answer.
Which of the following are true statements about the H-NMR spectrum of propan-2-ol?
1: There are four peaks in the spectrum (four hydrogen environments)
2: The integration trace will show a ratio of 1:1:6
3: There will be a doublet peak in the spectrum
The 3D image of propan-2-ol in shown below.
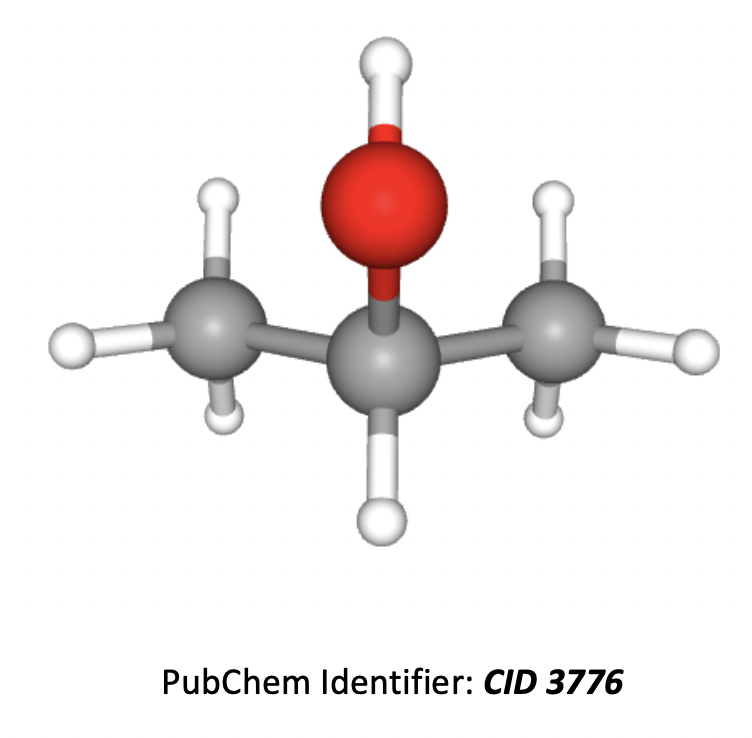
The hydrogens in the two CH3 groups on the ends of the molecule are all in the same environment (the molecule is symmetrical). So there are three hydrogen environments in total (not four).
The ratio of hydrogens in the environments is 1:1:6 (CH, OH and the two CH3 groups) so the integration trace will show a 1:1:6 ratio.
The H on the middle carbon will split the two CH3 (six hydrogen) peak into two, according to the n+1 rule. Therefore there will be a doublet in the spectrum.
Remember that OH hydrogens tend not to split or be split.
Therefore 2 and 3 only is the correct answer.
Which of the following compounds will include a quartet peak (amongst others) in their H-NMR spectra?
1: Ethane, CH3CH3
2: Ethanol, CH3CH2OH
3: Propanenitrile, CH3CH2CN
A quartet peak (a peak split into 4) occurs as a result of a hydrogen environment being split by three neighbouring hydrogens (next carbon) according to the n+1 rule.
Ethane is a symmetrical molecule and will have only one hydrogen environment; one singlet peak.
Ethanol and Propanenitrile both have three hydrogens in CH3 groups that will split the neighbouring hydrogen environment (CH2 group) into a quartet. Remember that the OH hydrogen in ethanol is not split and will not split.
Therefore both ethanol and propanenitrile will include a quartet peak in their H-NMR spectra.
Thus 2 and 3 only is the correct answer.
The IR spectrum and NMR spectrum below are both given by compound 'B'.
Which of these could be compound 'B'?
1: Propanoic acid, CH3CH2CO2H
2: Propanol, CH3CH2OH
3: Propanal, CH3CH2CHO
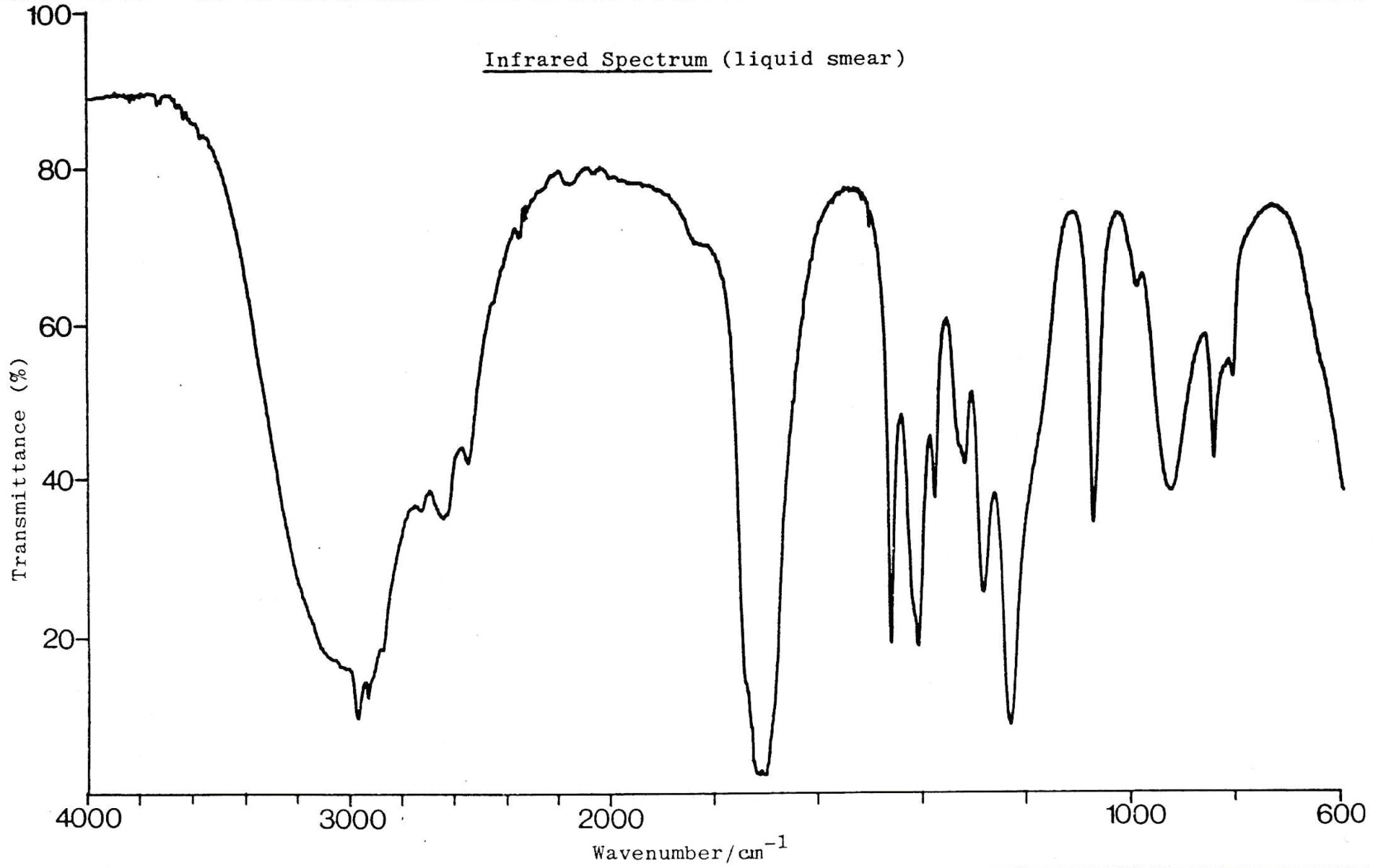
IR spectrum
NMR spectrum
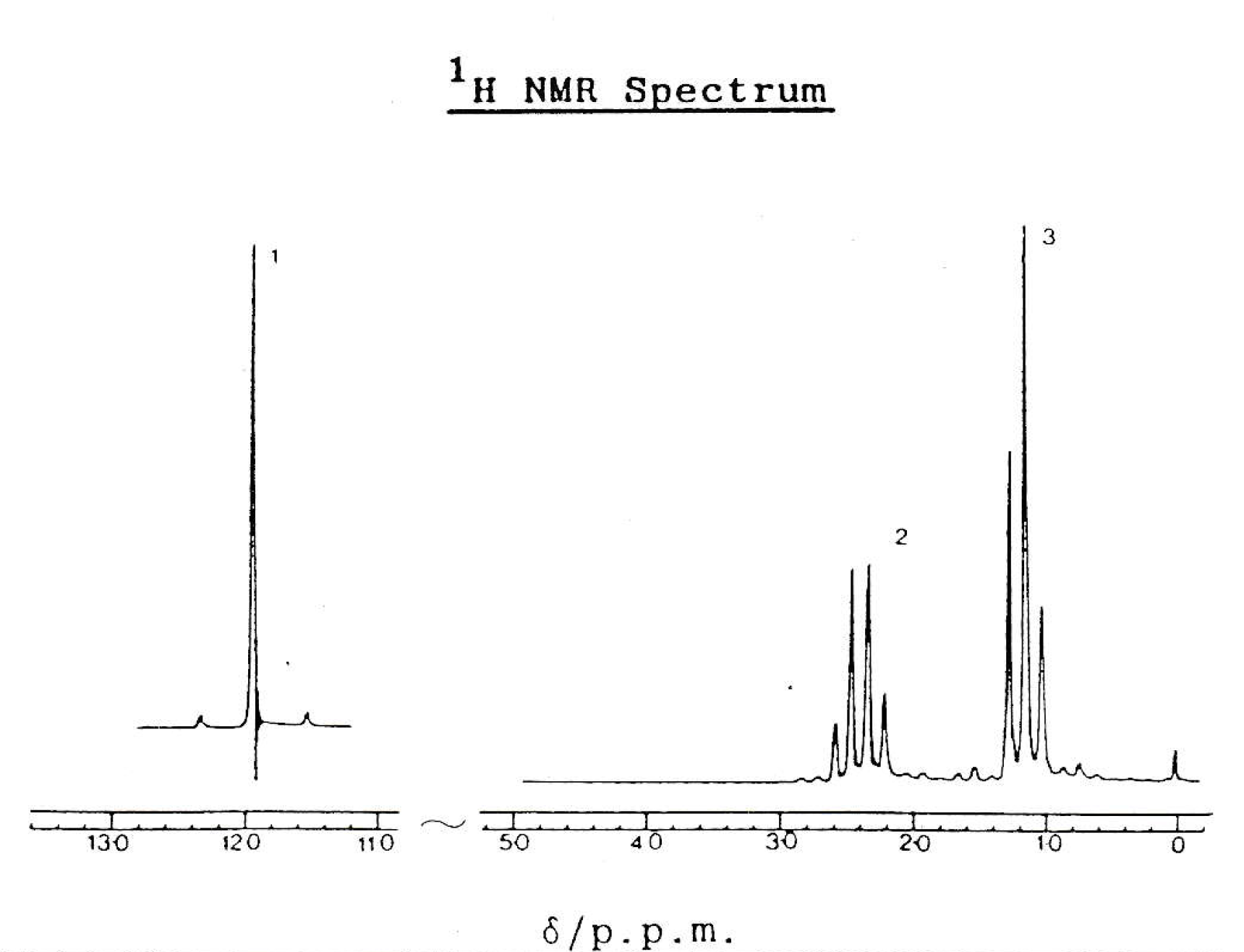
The IR spectrum shows a strong band around 1700cm−1. This suggests a C=O bond, so compound B cannot be propanol. Both propanoic acid and propanal have a C=O bond.
The IR spectrum also shows a characteristic 'overlapping' band at 3000cm−1. This suggests an O−H which is broad and rounded overlapping with the C−H band. This would suggest that the compound is a carboxylic acid.
The NMR spectrum shows three hydrogen environments, with hydrogen ratio of 1:2:3. This fits with all three compounds. The splitting pattern fits with propanol and propanoic acid (as we do not expect an O−H hydrogen to split or be split), although not with propanal (as we might expect the H on CHO to split and be split). However, the singlet O−H peak is shifted far down field, which is more characteristic of a carboxylic acid.
Besides, the IR has ruled out propanol due to the C=O so compound 'B' must be propanoic acid.
Therefore 1 only is the correct answer.
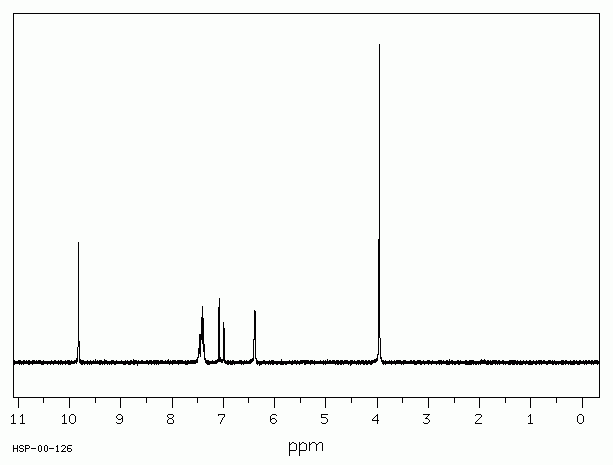
The H-NMR spectrum below is that of compound E.
Which of the following statements are true?
1: Compound E has two different hydrogen environments
2: The peak at approximately 1.2ppm is a triplet because there are three hydrogens in the environment
3: Compound E could be propanenitrile, CH3CH2CN
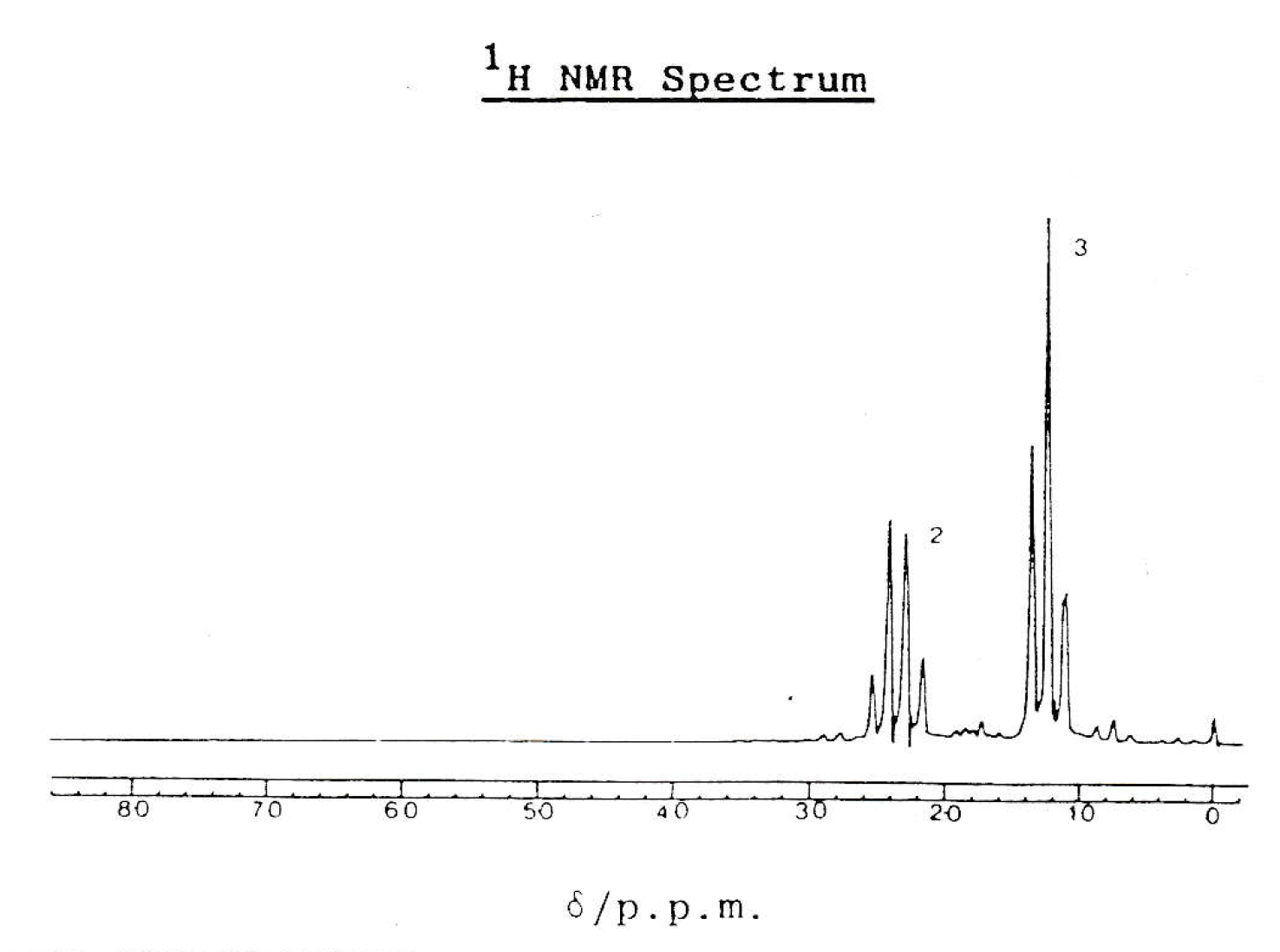
There are two peaks in the spectrum, so compound E does have two hydrogen environments.
The peak at approximately 1.2ppm is a triplet because it is split by two nearest neighbours (according to the n+1 rule); presumably a CH2 group on the next carbon. It is not split because it contains three hydrogens.
Propanenitrile has three hydrogens in the CH3 group that will split the neighbouring hydrogen environment (CH2 group) at 2.4ppm into a quartet, and vice versa to give the triplet at 1.2ppm. So compound E could be propanenitrile.
Thus 1 and 3 only is the correct answer.
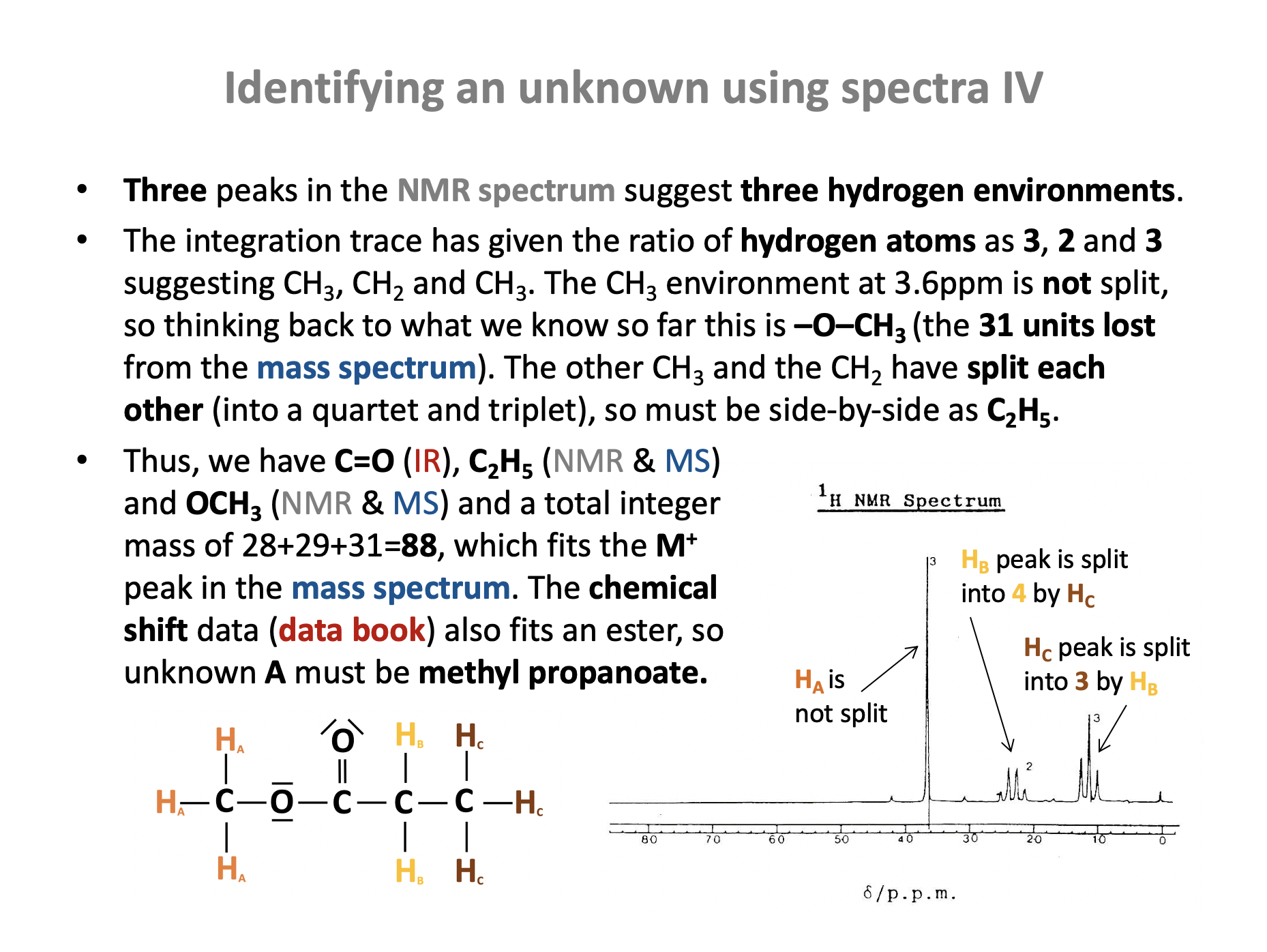
The mass spectrum below is that of compound 'K'. Compound K has a molecular formula of C4H8O2.
Which of the following is most likely to be compound K?
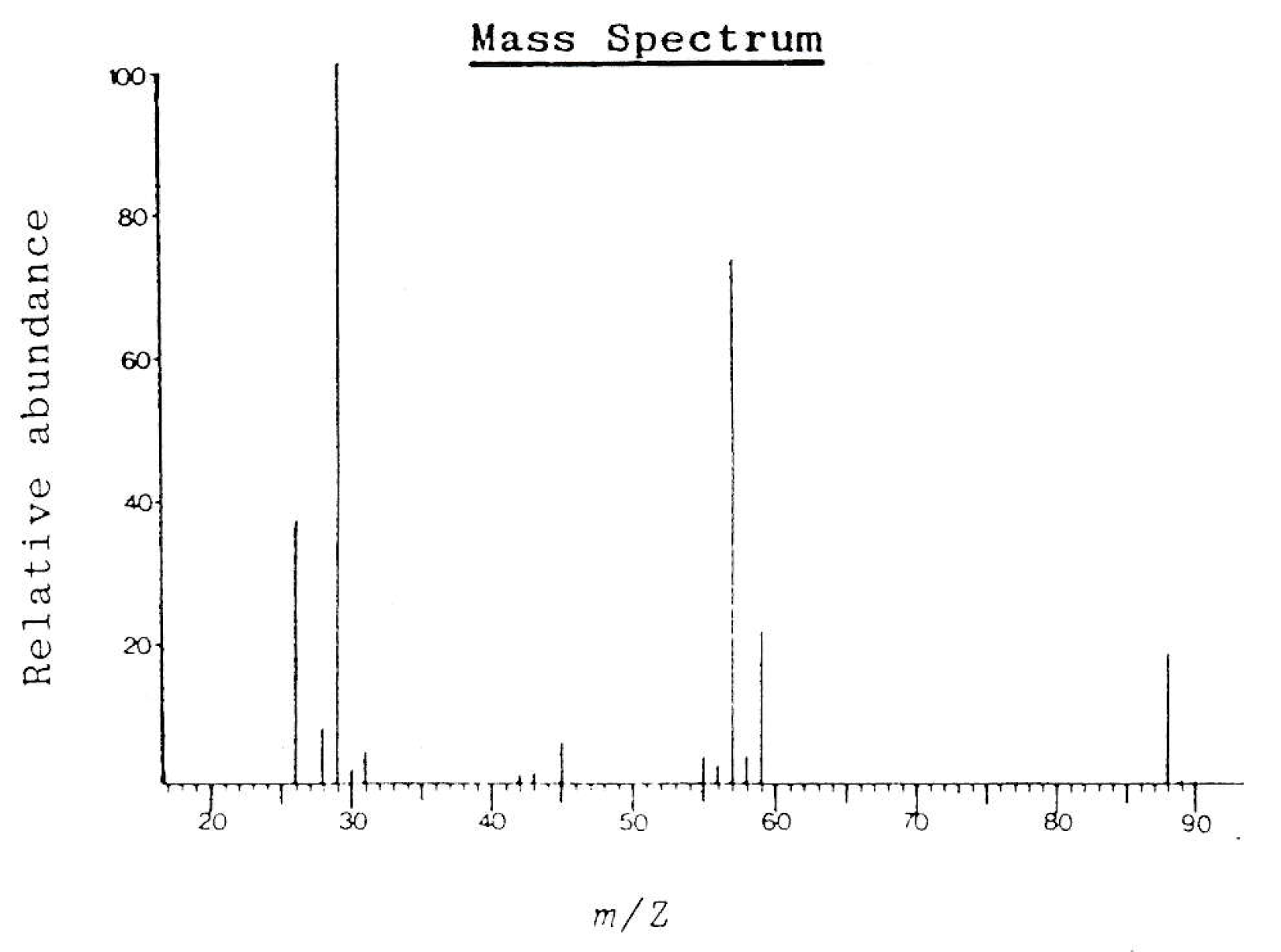
The molecular ion peak (M+) at 88 m/z corresponds to the integer mass for three compounds, but not to methyl ethanoate (and nor does the molecular formula) so that is eliminated.
The largest fragment ion peaks are at 29 m/z and 57 m/z.
Using the mass spectrum table in the data book, 29 m/z is likely to be [C2H5]+ (in theory it could also be [CHO]+, but that fragment is not viable in any of the four compounds given). C2H5 could not be a fragment of 2-methylpropanoic acid, so that may be eliminated.
The fragment ion at 57 m/z could be a fragment for methyl propanoate; it corresponds to [C2H5CO]+, but 57 m/z could not be a fragment for ethyl ethanoate: For ethyl ethanoate, we would expect significant (in terms of high abundance) fragments at 43 m/z ([CH3CO]+) and at 45 m/z ([OC2H5]+).
Therefore the correct answer is Methyl propanoate as this is most likely to be compound K.
Paper 1
Core (SL&HL): Measurement core (SL and HL) paper 1 questions
AHL (HL only): Measurement AHL (HL only) paper 1 questions
Paper 2
Core (SL&HL): Measurement core (SL & HL) paper 2 questions
AHL (HL only): Measurement AHL (HL only) paper 2 questions
How much of Spectroscopic identification II have you understood?













 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn