Ten suggestions for IAs using secondary data
With many schools around the world facing problems due to the pandemic, this page lists and discusses ten areas of chemistry where students could research and use secondary data from a variety of different sources to devise and address their own research question while access to a school laboratory is limited or completely unavailable.
Introduction
With many schools around the world now suffering from the pandemic or its after effects subscribing teachers have been asking me (and also others on Facebook and My IB etc.) if I can come up with some suggestions for IAs that can while laboratory access is curtailed. In some ways this goes against the spirit of the IB as it is quite clear in the answer given to Question 25 in the IB’s frequently asked questions that “Students should be coming up with their own ideas for their IA although some guidance and support can be provided.” Nevertheless these are strange times and I feel that it is reasonable to give some help. Firstly though it is worth considering that students who are (hopefully!) sitting their final IB Diploma exams in May 2022 may still have time to carry out some laboratory work as many students do not start work on their IA until their second year. It may also be possible for your school to negotiate with national and local authorities to be able to open your chemistry laboratory for one or two days so that students can do laboratory work whilst still retaining social distancing. However, if this is not possible, then the following suggestions might be useful. Note that they all involve the students finding their own secondary data rather than generating it through simulations. Simulations may be good for illustrating concepts and practical techniques etc. but they are generally written to give expected results. To my way of thinking there is more possibility for a student to show creativity and personal input if they seek out their own secondary data from a variety of different sources to address their research question. I have listed some good data sources on the page on Databases including one excellent source from Depth-First for 64 different free data bases, which although it dates from 2011 is still valid.
You will see from the list below that the data required is easily accessible. I have already touched upon some of the ideas in other areas of this site. Ideally the ideas will be ‘thrown up’ by you as you teach a topic (e.g. when covering VSEPR theory it is worth adding that although it works well for many simple molecules there are others where it would appear to give a misleading result – maybe this would be an interesting topic for an IA….) rather than just giving students the list when it comes to choosing a topic for their IA. Although the titles below are framed as questions they cover broad areas and students (with your guidance) will need to arrive at their own focused research question.
In addition to the information and ideas given here I strongly recommend you also look at two videos prepared by James Midgley who is the Head of Year 12 at the Australian International School in Singapore and an IA moderator. The first discusses how to research and attain high marks.
![]() How to smash your IA - The pandemic edition
How to smash your IA - The pandemic edition
The second video is of a zoom webinar that James conducted taking you through two genuine data based IAs (one of which gets 24/24) and showing you exactly how he has marked them. I suggest you fast forward to 18 minutes as that is when the webinar actually starts.
It is also well worth loking at a site put together by Jon Chui who works at the United World College of Hong Kong. This is entitled IBDP Chem: Data Analysis Skills and gives advice and information about how to find and analyse secondary data.
Areas for research
1. How do different side groups affect the precise position of IR absorptions due to a particular functional group?
For example, the IR absorption due to the carbonyl group is particularly sharp. The IB data booklet gives a value of between 1700 and 1750 cm−1.
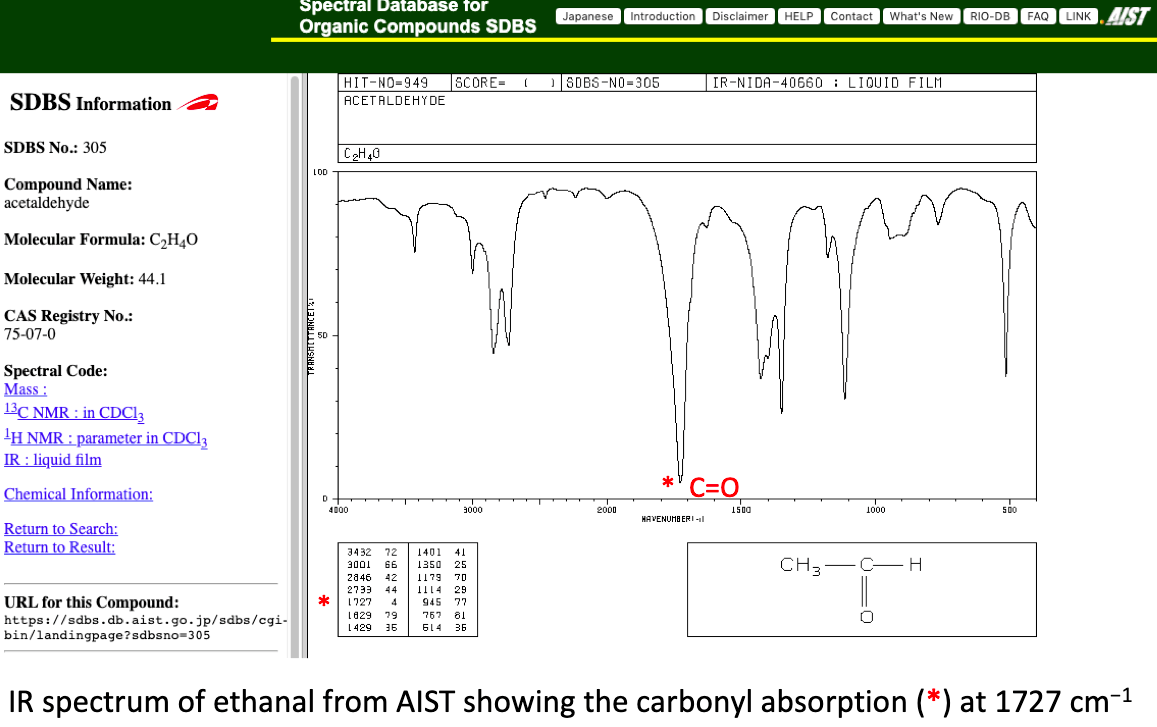
Ethanal, CH3CHO absorbs at 1727 cm−1 and propanal at 1733 cm−1. Is there a trend of increasing wavenumber as the size of the R group increases? (Reference for values : AIST)
2. What impact have the efforts to combat the Covid-19 pandemic had on levels of nitrogen dioxide in the atmosphere?
There is now a lot of data on how nitrogen dioxide levels have changed since the lockdown due to Covid-19. For example see the World Health Organization fact sheet and also an article on CNN from which the two images of Delhi, one on 3 November 2019 and the other on 30 March 2020 were taken.
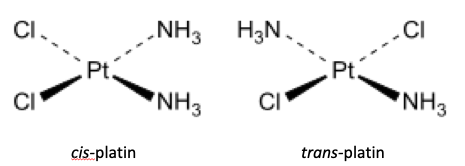
3. How important is stereoisomerism in transition metal chemistry?
The IB syllabus only really covers examples of stereochemistry in organic molecules but inorganic molecules can also show stereoisomerism (see the HL page on Coloured complexes). These can have interesting and unique properties, for example: Why is cis-platin effective against certain cancers whereas trans-platin is not?
4. How rigorous is the IB Diploma chemistry syllabus as it is currently laid out?
Even though it has gone through several revisions and versions initially on the OCC and now on My IB there are still inconsistencies and problems with the IB syllabus as it is currently laid out. A good student could analyse the syllabus and make recommendations for changes. For example:
a) Assuming SL students understand AHL material
There are several examples on the syllabus where a knowledge of AHL is assumed for SL students, e.g.

SL students are directed to a table in the data booklet that lists the pKa values of various acids but neither Ka nor pKa are on the SL syllabus. They are covered in the AHL sub-topic 18.2.
b) Incorrect chemistry and/or NOS
There are several examples of wrong chemistry and wrong NOS/TOK statements. For example, in 14.1 it states

In the 'Guidance' in the same sub-topic it does correctly state that the linear combination of atomic orbitals to form molecular orbitals should be covered but combination is not overlap. Atomic orbitals combine either constructively to give bonding molecular orbitals or destructively to give anti-bonding molecular orbitals - they do not 'overlap'.

Occam's razor basically states that when presented with different hypotheses to reach the same conclusion the simplest hypothesis is the most likely one. The pH scale has nothing to do with Occam's razor, it is simply expressing the hydrogen ion concentration in a logarithmic scale.
c) Inconsistent terminology
Consider the following two statements from the guide:
.png)
The first statement is from 5.1 in the Chemistry guide the second is from 5.2. Both should show H in change in enthalpy in italics.
5. How do the values of ebullioscopic and cryoscopic constants relate to chemical structure and/or polarity?
Colligative properties are no longer on the IB syllabus but can provide a useful area to research. The values of both elevation of boiling point constants (ebullioscopic constants) and depression of freezing point constants (cryoscopic constants) can vary by considerable amounts. For example, why is the ebullioscopic constant of methanol 0.86 oC/molal whereas the value for iodomethane is 4.31 oC/molal? (Reference: LibraTexts).
6. How useful were the changes made to the 2016 IB data booklet compared to the 2009 IB data booklet?
Considerable changes were made to the IB data book for the current syllabus. It could prove interesting to analyse these changes and see how useful (or otherwise) they are. For example, if the values for the atomic radius of metals such as sodium given in the 2016 data booklet are used to calculate the density of sodium they give completely the wrong answer whereas those given in the 2009 data booklet give the correct answer - see Superconducting metals & X-ray crystallography.
7. How consistent is the current IB data booklet?
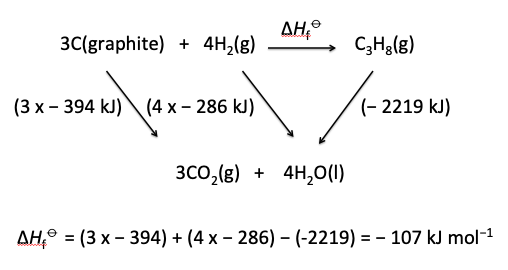
In the same way that the Chemistry guide can be analysed so can the current IB data booklet. For example, in Section 2 the ionic product of water at 298 K has units of mol2 dm-6 whereas the values for the ionization constants for water in Section 23 and the values for solubility products in Section 32 have no units. Even though no uncertainties are given the values in different sections sometimes do not match. For example in Section 12 the enthalpy of formation of propane is given as − 105 kJ mol−1. Hess's Law can be used to calculate this value using the enthalpies of combustion for graphite, hydrogen and propane in Section 13. These values are given as − 394, − 286 and − 2219 respectively. Using these values ΔHf⦵(propane) = (3 x − 394) + (4 x − 286) − (−2219) = − 107 kJ mol−1 which is not the same as the value given in Section 12.
8. Are the concepts of equivalent weight and normality still of any use?

Sixty years ago all chemists used equivalent weight and normality. Why was this once an integral part of stoichiometric calculations and yet now is no longer taught in schools? Why has it disappeared and what has replaced it? Very few modern chemists use (or even understand) the terms and yet N (normality) rather than M (molarity) is still often used when solutions are bought (see image above).
9. How useful is VSEPR theory at predicting bond angles?
VSEPR theory is very good at explaining the shape and bond angles of methane, ammonia and water but what about H3O+ where the bond angle has been measured to be 113.6o? Other bond angles to consider include those in PCl3, PH3, H2S and SCl2 - see Covalent structures (2).
10. Why do alkyl groups have a positive inductive effect?
Markovnikov’s rule for the addition of protic acids, HX to asymmetrical alkenes can be explained by the stability of the resulting carbocation (see the page on Electrophilic addition). Tertiary carbocations are the most stable due to the positive inductive effect of the alkyl groups which ‘push’ electrons towards the positive carbon atom thus stabilizing it. The strength of this effect is apparent when looking at the pKa values of carboxylic acids.
.png)
pKa values of carboxylic acids and electronegativity values
of H, C, and I from the IB data booklet
Iodoethanoic acid has a pKa of 3.18. Iodine has an electronegativity value of 2.7 making it virtually the same as carbon (2.6). If the iodine atom is substituted by a hydrogen atom (electronegativity value 2.2) then the resulting acid, ethanoic acid is considerably weaker with a pKa value of 4.76. This is said to be due to the positive inductive effect of the methyl group. What is surprising is that if the methyl group itself is substituted by a relatively electropositive hydrogen atom to give methanoic acid it might be expected that the acid would be even weaker (as the electronegativity of the atom bonded directly to the carboxylic carbon atom has decreased from 2.6 to 2.2) whereas in fact the pKa of methanoic acid is 3.75. This means that methanoic acid is ten times stronger than ethanoic acid.
Using data from Sections 8 and 21 of the IB data booklet and from other sources students could explore the factors affecting the strengths of carboxylic acids and try to determine what other factors affect the strength apart from electronegativity values.

 IB Docs (2) Team
IB Docs (2) Team